Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
The decomposition of SOCl2 is first-order in SOCl2.If the half-life for the reaction is 4.1 hr,how long would it take for the concentration of SOCl2 to drop from 0.36 M to 0.045 M?
(Multiple Choice)
4.8/5  (32)
(32)
The rate law cannot be predicted from the stoichiometry of a reaction.
(True/False)
4.9/5  (26)
(26)
In the gas phase at 500.°C,cyclopropane reacts to form propene in a first-order reaction.The figure below shows the concentration of cyclopropane plotted versus time.Use the graph to calculate approximate values of
a.the rate of the reaction,600.seconds after the start.
b.the half-life of the reaction,t1/2. 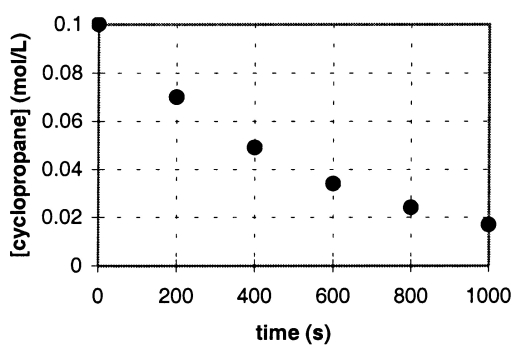
(Essay)
4.8/5  (32)
(32)
For the reaction
2A + B + 2C D + E
The following initial rate data was collected at constant temperature.Determine the correct rate law for this reaction.All units are arbitrary.

(Multiple Choice)
4.9/5  (34)
(34)
Which of the following sets of units could be appropriate for a zero-order rate constant?
(Multiple Choice)
4.9/5  (44)
(44)
The rate of a reaction is determined by the rate of the fastest step in the mechanism.
(True/False)
4.9/5  (30)
(30)
The elementary reaction HBr(g)+ Br(g) H(g)+ Br2(g)is endothermic.
a.Would you expect the rate constant for the back reaction to be smaller or larger than that for the forward reaction? Explain,briefly.
b.Draw a fully-labeled reaction energy diagram for this reaction,showing the locations of the reactants,products and transition state.
(Essay)
5.0/5  (38)
(38)
A first-order reaction has a half-life of 20.0 minutes.Starting with 1.00 1020 molecules of reactant at time t = 0,how many molecules remain unreacted after 100.0 minutes?
(Multiple Choice)
4.7/5  (38)
(38)
The half-life of a first-order reaction does not depend on the initial concentration of reactant.
(True/False)
4.8/5  (39)
(39)
The gas-phase conversion of 1,3-butadiene to 1,5-cyclooctadiene,2C4H6 C8H12 was studied,providing data for the plot shown below,of 1/[butadiene] versus time.
a.Explain how this plot confirms that the reaction is second order.
b.Calculate the second-order rate constant,k.
c.Determine the initial concentration of 1,3-butadiene in this experiment. ![The gas-phase conversion of 1,3-butadiene to 1,5-cyclooctadiene,2C<sub>4</sub>H<sub>6</sub> \to C<sub>8</sub>H<sub>12</sub> was studied,providing data for the plot shown below,of 1/[butadiene] versus time. a.Explain how this plot confirms that the reaction is second order. b.Calculate the second-order rate constant,k. c.Determine the initial concentration of 1,3-butadiene in this experiment.](https://storage.examlex.com/TB5832/11ea8a63_05f3_cae8_9a87_5773a962773e_TB5832_00.jpg)
(Essay)
4.9/5  (29)
(29)
When the reaction A B + C is studied,a plot of ln[A]t vs.time gives a straight line with a negative slope.What is the order of the reaction?
(Multiple Choice)
4.9/5  (33)
(33)
Consider the reaction
2NH3(g) N2(g)+ 3H2(g)
If the rate [H2]/ t is 0.030 mol L-1 s-1,then [NH3]/ t is
(Multiple Choice)
4.7/5  (42)
(42)
The rate law for the reaction 3A 2B is rate = k[A] with a rate constant of 0.0447 hr-1.What is the half-life of the reaction?
(Multiple Choice)
4.8/5  (31)
(31)
The units of the rate constant depend on the order of the reaction.
(True/False)
4.9/5  (37)
(37)
A study of the decomposition reaction 3RS2 3R + 6S yields the following initial rate data
 What is the rate constant for the reaction?
What is the rate constant for the reaction?
(Multiple Choice)
4.7/5  (32)
(32)
Tetrafluoroethylene,C2F4,can be converted to octafluorocyclobutane which can be used as a refrigerant or an aerosol propellant.A plot of 1/[C2F4] vs.time gives a straight line with a slope of 0.0448 L mol-1s-1.What is the rate law for this reaction?
(Multiple Choice)
4.7/5  (27)
(27)
In the gas phase at 500.°C,cyclopropane reacts to form propene in a first-order reaction.The figure shows the natural logarithm of the concentration of cyclopropane (in mol/L)plotted versus time.
a.Explain how this plot confirms that the reaction is first order.
b.Calculate the first-order rate constant,k.
c.Determine the initial concentration of cyclopropane in this experiment. 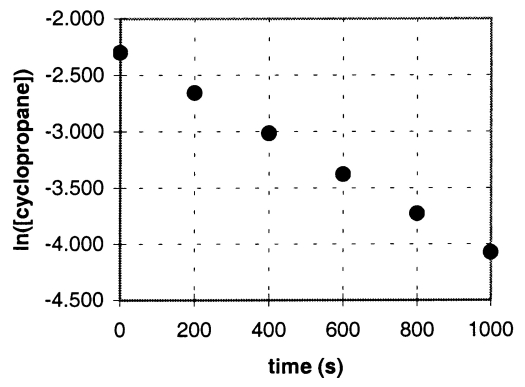
(Essay)
4.8/5  (36)
(36)
The greater the energy of activation,Ea,the faster will be the reaction.
(True/False)
4.8/5  (32)
(32)
The rate law for the reaction 3A C is
Rate = 4.36 10-2 L mol-1 hr-1[A]2
What is the half-life for the reaction if the initial concentration of A is 0.250 M?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 41 - 60 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)