Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution.
H+ + H2O2  H2O+-OH (rapid equilibrium)
H2O+-OH + Br- HOBr + H2O (slow)
HOBr + H+ + Br- Br2 + H2O (fast)
What is the overall reaction equation for this process?
H2O+-OH (rapid equilibrium)
H2O+-OH + Br- HOBr + H2O (slow)
HOBr + H+ + Br- Br2 + H2O (fast)
What is the overall reaction equation for this process?
(Multiple Choice)
4.8/5  (33)
(33)
The units of the rate of reaction depend on the order of the reaction.
(True/False)
4.9/5  (34)
(34)
Which one of the following sets of units is appropriate for a third-order rate constant?
(Multiple Choice)
4.8/5  (34)
(34)
Is a bimolecular reaction necessarily second-order? Is a second-order reaction necessarily bimolecular? Answer,with explanations and clarifications.
(Essay)
5.0/5  (33)
(33)
The rate law for the rearrangement of CH3NC to CH3CN at 800 K is rate = (1300 s-1)[CH3NC].What is the half-life for this reaction?
(Multiple Choice)
4.8/5  (34)
(34)
The compound RX3 decomposes according to the equation
3RX3 \(\to\0 R + R2X3 + 3X2
In an experiment the following data were collected for the decomposition at 100°C.What is the average rate of reaction over the entire experiment?
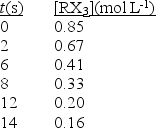
(Multiple Choice)
4.8/5  (26)
(26)
Consider the following mechanism for the oxidation of bromide ions by hydrogen peroxide in aqueous acid solution.
H+ + H2O2  H2O+-OH (rapid equilibrium)
H2O+-OH + Br- HOBr + H2O (slow)
HOBr + H+ + Br- Br2 + H2O (fast)
Which of the following rate laws is consistent with the mechanism?
H2O+-OH (rapid equilibrium)
H2O+-OH + Br- HOBr + H2O (slow)
HOBr + H+ + Br- Br2 + H2O (fast)
Which of the following rate laws is consistent with the mechanism?
(Multiple Choice)
4.8/5  (37)
(37)
When the reaction A B + C is studied,a plot 1/[A]t vs.time gives a straight line with a positive slope.What is the order of the reaction?
(Multiple Choice)
5.0/5  (39)
(39)
The decomposition of dinitrogen pentaoxide to nitrogen dioxide and oxygen follows first-order kinetics and has an activation energy of 102 kJ/mol.By what factor will the fraction of collisions with energy greater than or equal to the activation energy increase if the reaction temperature goes from 30°C to 60°C?
(Multiple Choice)
4.8/5  (32)
(32)
The rate constant for the reaction 3A 4B is 6.00 10-3 L mol-1min-1.How long will it take the concentration of A to drop from 0.75 M to 0.25 M?
(Multiple Choice)
4.9/5  (46)
(46)
The reaction A B is first-order overall and first-order with respect to the reactant A.The result of doubling the initial concentration of A will be to
(Multiple Choice)
4.8/5  (38)
(38)
A B
At very low pressures many such reactions occur by the following mechanism:
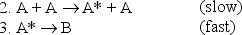 (A* represents a molecule with sufficient energy to overcome the activation energy barrier. )
a.Which of the three reactions above is/are elementary?
b.Where appropriate,identify the molecularity of the reactions.
c.Show that the proposed mechanism is consistent with reaction 1,the observed reaction.
d.Given the mechanism above,suggest a likely rate law for reaction (1).
(A* represents a molecule with sufficient energy to overcome the activation energy barrier. )
a.Which of the three reactions above is/are elementary?
b.Where appropriate,identify the molecularity of the reactions.
c.Show that the proposed mechanism is consistent with reaction 1,the observed reaction.
d.Given the mechanism above,suggest a likely rate law for reaction (1).
(Essay)
4.8/5  (31)
(31)
The half-life of a second-order reaction does not depend on the initial concentration of reactant.
(True/False)
4.8/5  (43)
(43)
A chemical reaction of the general type
A 2B
is first-order,with a rate constant of 1.52 10-4 s-1.
a.Calculate the half-life of A.
b.Assuming the initial concentration of A is 0.067 mol L-1,calculate the time needed for the concentration to fall to 0.010 mol L-1.
(Essay)
4.9/5  (33)
(33)
A reactant R is being consumed in a first-order reaction.What fraction of the initial R is consumed in 4.0 half-lives?
(Multiple Choice)
4.7/5  (35)
(35)
Which one of the following sets of units is appropriate for a second-order rate constant?
(Multiple Choice)
4.8/5  (40)
(40)
Consider the general reaction
5Br-(aq)+ BrO3-(aq)+ 6H+(aq) 3Br2(aq)+ 3H2O(aq)
For this reaction,the rate when expressed as [Br2]/ t is the same as
(Multiple Choice)
4.8/5  (41)
(41)
You are studying the rate of the reaction 2A B and have obtained measurements of the concentration of A at times t = 100,200,300,...... ,1000 seconds from the start of the reaction.Carefully describe how you would plot a graph and use it to
a.prove that the reaction is second-order with respect to A.
b.determine the second-order rate constant k.
(Essay)
4.8/5  (37)
(37)
Briefly list the features/properties common to all catalysts and how they work.Draw a labeled reaction energy diagram as part of your answer.
(Essay)
4.9/5  (28)
(28)
The kinetics of the decomposition of dinitrogen pentaoxide is studied at 50°C and at 75°C.Which of the following statements concerning the studies is correct?
(Multiple Choice)
4.8/5  (43)
(43)
Showing 21 - 40 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)