Exam 17: Equilibrium: the Extent of Chemical Reactions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Write the mass-action expression,Qc ,for the following chemical reaction.
MgO(s)+ SO2(g)+ 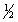 O2(g)
O2(g)  MgSO4(s)
MgSO4(s)
(Multiple Choice)
5.0/5  (34)
(34)
For a solution equilibrium,a change in concentration of a reactant or product does not change Kc.
(True/False)
4.8/5  (34)
(34)
a.State Le Chatelier's principle
b.The following reaction is at equilibrium in a closed container:
 What effects,if any,will the following actions have on the position of equilibrium? In each case,state the direction of any shift in equilibrium,and give your reasons in one sentence.
(i)adding more Fe(OH)3
(ii)raising the temperature
(iii)adding a catalyst
What effects,if any,will the following actions have on the position of equilibrium? In each case,state the direction of any shift in equilibrium,and give your reasons in one sentence.
(i)adding more Fe(OH)3
(ii)raising the temperature
(iii)adding a catalyst
(Essay)
4.7/5  (42)
(42)
The following reaction is at equilibrium at a pressure of 1 atm,in a closed container.
 Which,if any,of the following actions will decrease the concentration of CO2 gas present at equilibrium?
Which,if any,of the following actions will decrease the concentration of CO2 gas present at equilibrium?
(Multiple Choice)
4.9/5  (42)
(42)
At 25°C,the equilibrium constant Kc for the reaction in the solvent CCl4
2BrCl  Br2 + Cl2
is 0.141.If the initial concentration of chlorine is 0.0300 M and of bromine monochloride is 0.0200 M,what is the equilibrium concentration of bromine?
Br2 + Cl2
is 0.141.If the initial concentration of chlorine is 0.0300 M and of bromine monochloride is 0.0200 M,what is the equilibrium concentration of bromine?
(Multiple Choice)
4.9/5  (36)
(36)
When 0.152 mol of solid PH3BCl3 is introduced into a 3.0 L container at a certain temperature,8.44 10-3 mol of PH3 is present at equilibrium:
PH3BCl3(s)  PH3(g)+ BCl3(g)
Construct a reaction table for the process,and use it to calculate Kc at this temperature.
PH3(g)+ BCl3(g)
Construct a reaction table for the process,and use it to calculate Kc at this temperature.
(Essay)
4.9/5  (38)
(38)
Compounds A,B,and C react according to the following equation.
3A(g)+ 2B(g) ![Compounds A,B,and C react according to the following equation. 3A(g)+ 2B(g) 2C(g) At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M,[B] = 1.23 M,and [C] = 1.75 M.What is the value of K<sub>c</sub> for this reaction?](https://storage.examlex.com/TB5832/11ea8a63_05f9_227e_9a87_0f734142eee0_TB5832_11.jpg) 2C(g)
At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M,[B] = 1.23 M,and [C] = 1.75 M.What is the value of Kc for this reaction?
2C(g)
At 100°C a mixture of these gases at equilibrium showed that [A] = 0.855 M,[B] = 1.23 M,and [C] = 1.75 M.What is the value of Kc for this reaction?
(Multiple Choice)
4.8/5  (30)
(30)
About half of the sodium carbonate produced is used in making glass products because it lowers the melting point of sand,the major component of glass.When sodium carbonate is added to water it hydrolyses according to the following reactions.
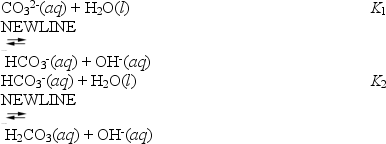 These can be combined to yield
These can be combined to yield
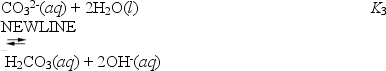 What is the value of K3?
What is the value of K3?
(Multiple Choice)
4.8/5  (35)
(35)
Sodium hydrogen carbonate decomposes above 110°C to form sodium carbonate,water,and carbon dioxide.
2NaHCO3(s)  Na2CO3(s)+ H2O(g)+ CO2(g)
One thousand grams of sodium hydrogen carbonate are added to a reaction vessel,the temperature is increased to 200°C,and the system comes to equilibrium.What happens in this system if another 50 g of sodium carbonate are now added?
Na2CO3(s)+ H2O(g)+ CO2(g)
One thousand grams of sodium hydrogen carbonate are added to a reaction vessel,the temperature is increased to 200°C,and the system comes to equilibrium.What happens in this system if another 50 g of sodium carbonate are now added?
(Multiple Choice)
4.8/5  (33)
(33)
At 850°C,the equilibrium constant Kp for the reaction
C(s)+ CO2(g)  2CO(g)
Has a value of 10.7.If the total pressure in the system at equilibrium is 1.000 atm,what is the partial pressure of carbon monoxide?
2CO(g)
Has a value of 10.7.If the total pressure in the system at equilibrium is 1.000 atm,what is the partial pressure of carbon monoxide?
(Multiple Choice)
4.9/5  (46)
(46)
Consider the reactions of cadmium with the thiosulfate anion.
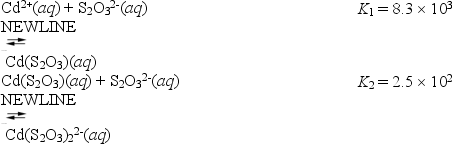 What is the value for the equilibrium constant for the following reaction?
Cd2+(aq)+ 2S2O32-(aq)
What is the value for the equilibrium constant for the following reaction?
Cd2+(aq)+ 2S2O32-(aq)  Cd(S2O3)22-(aq)
Cd(S2O3)22-(aq)
(Multiple Choice)
4.8/5  (39)
(39)
Hydrogen bromide will dissociate into hydrogen and bromine gases.
 What effect will a temperature increase of 50°C have on this system at equilibrium?
What effect will a temperature increase of 50°C have on this system at equilibrium?
(Multiple Choice)
4.8/5  (36)
(36)
Write the expression for Kc and Kp for the reaction
PH3BCl3(s)  PH3(g)+ BCl3(g)
PH3(g)+ BCl3(g)
(Essay)
4.7/5  (36)
(36)
Nitrogen dioxide decomposes according to the reaction
2NO2(g)  2NO(g)+ O2(g)
Where Kp = 4.48 10-13 at 25°C.What is the value for Kc?
2NO(g)+ O2(g)
Where Kp = 4.48 10-13 at 25°C.What is the value for Kc?
(Multiple Choice)
4.9/5  (34)
(34)
Ammonia is synthesized in the Haber process:
N2(g)+ 3H2(g)  2NH3(g)
Kp for this reaction is 1.49 10-5 atm-2 at 500.°C.Calculate Kc at this temperature.
2NH3(g)
Kp for this reaction is 1.49 10-5 atm-2 at 500.°C.Calculate Kc at this temperature.
(Essay)
4.9/5  (38)
(38)
Which of the following has an effect on the magnitude of the equilibrium constant?
(Multiple Choice)
4.7/5  (36)
(36)
The equilibrium constant,Kp,has a value of 6.5 10-4 at 308 K for the reaction of nitrogen monoxide with chlorine.
2NO(g)+ Cl2(g)  2NOCl(g)
What is the value of Kc?
2NOCl(g)
What is the value of Kc?
(Multiple Choice)
4.9/5  (38)
(38)
Although a system may be at equilibrium,the rate constants of the forward and reverse reactions will in general be different.
(True/False)
4.8/5  (34)
(34)
Methanol can be synthesized by combining carbon monoxide and hydrogen.
CO(g)+ 2H2(g)  CH3OH(g)
A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm.What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
CH3OH(g)
A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm.What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
(Multiple Choice)
4.9/5  (47)
(47)
Nitric oxide is formed in automobile exhaust when nitrogen and oxygen in air react at high temperatures.
N2(g)+ O2(g)  2NO(g)
The equilibrium constant Kp for the reaction is 0.0025 at 2127°C.If a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium,what will be the equilibrium partial pressure of nitrogen?
2NO(g)
The equilibrium constant Kp for the reaction is 0.0025 at 2127°C.If a container is charged with 8.00 atm of nitrogen and 5.00 atm of oxygen and the mixture is allowed to reach equilibrium,what will be the equilibrium partial pressure of nitrogen?
(Multiple Choice)
4.7/5  (38)
(38)
Showing 21 - 40 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)