Exam 17: Equilibrium: the Extent of Chemical Reactions
Exam 1: Keys to the Study of Chemistry66 Questions
Exam 2: The Components of Matter91 Questions
Exam 3: Stoichiometry of Formulas and Equations90 Questions
Exam 4: Three Major Classes of Chemical Reactions84 Questions
Exam 5: Gases and the Kinetic-Molecular Theory93 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change71 Questions
Exam 7: Quantum Theory and Atomic Structure72 Questions
Exam 8: Electron Configuration and Chemical Periodicity70 Questions
Exam 9: Models of Chemical Bonding60 Questions
Exam 10: The Shapes of Molecules94 Questions
Exam 11: Theories of Covalent Bonding49 Questions
Exam 12: Intermolecular Forces: Liquids,solids,and Phase Changes89 Questions
Exam 13: The Properties of Solutions73 Questions
Exam 14: The Main-Group Elements: Applying Principles of Bonding and Structure58 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon95 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions76 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions85 Questions
Exam 18: Acid-Base Equilibria90 Questions
Exam 19: Ionic Equilibria in Aqueous Systems96 Questions
Exam 20: Thermodynamics: Entropy, free Energy, and the Direction of Chemical Reactions84 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work97 Questions
Exam 22: The Transition Elements and Their Coordination Compounds72 Questions
Exam 23: Nuclear Reactions and Their Applications75 Questions
Select questions type
Write the mass-action expression,Qc,for the following chemical reaction.
Fe3+(aq)+ 3OH-(aq)  Fe(OH)3(s)
Fe(OH)3(s)
(Multiple Choice)
4.7/5  (27)
(27)
The reaction of nitric oxide to form dinitrogen oxide and nitrogen dioxide is exothermic.
3NO(g)  N2O(g)+ NO2(g)+ heat
What effect will be seen if the temperature of the system at equilibrium is raised by 25°C?
N2O(g)+ NO2(g)+ heat
What effect will be seen if the temperature of the system at equilibrium is raised by 25°C?
(Multiple Choice)
4.8/5  (33)
(33)
Write the mass-action expression,Qc,for the following chemical reaction.
3ClO2-(aq)  2ClO3-(aq)+ Cl-(aq)
2ClO3-(aq)+ Cl-(aq)
(Multiple Choice)
4.7/5  (27)
(27)
Consider the following two equilibria and their respective equilibrium constants:
(1)NO(g)+ 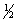 O2(g)
O2(g)  NO2(g)
(2)2NO2(g)
NO2(g)
(2)2NO2(g)  2NO(g)+ O2(g)
Which one of the following is the correct relationship between the equilibrium constants K1 and K2?
2NO(g)+ O2(g)
Which one of the following is the correct relationship between the equilibrium constants K1 and K2?
(Multiple Choice)
4.8/5  (27)
(27)
The following reaction is at equilibrium in a sealed container.
 Which,if any,of the following actions will increase the value of the equilibrium constant,Kc?
Which,if any,of the following actions will increase the value of the equilibrium constant,Kc?
(Multiple Choice)
4.9/5  (27)
(27)
Write the mass-action expression,Qc,for the following chemical reaction.
Zn(s)+ 2Ag+(aq)  Zn2+(aq)+ 2Ag(s)
Zn2+(aq)+ 2Ag(s)
(Multiple Choice)
4.7/5  (35)
(35)
Consider the equilibrium
H2(g)+ Br2(g)  2HBr(g)
To a 20.0 L flask are added 0.100 moles of H2 and 0.200 moles of HBr.The equilibrium constant for this reaction is 989.Calculate the number of moles of Br2 in the flask when equilibrium is established.Make any reasonable approximation,clearly stating what that approximation is.
2HBr(g)
To a 20.0 L flask are added 0.100 moles of H2 and 0.200 moles of HBr.The equilibrium constant for this reaction is 989.Calculate the number of moles of Br2 in the flask when equilibrium is established.Make any reasonable approximation,clearly stating what that approximation is.
(Essay)
4.9/5  (44)
(44)
A container was charged with hydrogen,nitrogen,and ammonia gases at 120°C and the system was allowed to reach equilibrium.What will happen if the volume of the container is increased at constant temperature?
3H2(g)+ N2(g)  2NH3(g)
2NH3(g)
(Multiple Choice)
4.9/5  (23)
(23)
Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide.
NH4I(s)  NH3(g)+ HI(g)
At 400°C,Kp = 0.215.Calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400°C.
NH3(g)+ HI(g)
At 400°C,Kp = 0.215.Calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400°C.
(Multiple Choice)
4.9/5  (42)
(42)
The reaction system
POCl3(g)  POCl(g)+ Cl2(g)
Is at equilibrium.Which of the following statements describes the behavior of the system if POCl is added to the container?
POCl(g)+ Cl2(g)
Is at equilibrium.Which of the following statements describes the behavior of the system if POCl is added to the container?
(Multiple Choice)
4.9/5  (37)
(37)
Changing the amount of reactant or product in an equilibrium reaction will always change the equilibrium position,regardless of the physical state of the substance involved.
(True/False)
4.9/5  (47)
(47)
There is a direct correlation between the speed of a reaction and its equilibrium constant.
(True/False)
4.9/5  (38)
(38)
In order to write the correct mass-action expression for a reaction one must
(Multiple Choice)
4.9/5  (26)
(26)
Nitrogen dioxide can dissociate to nitric oxide and oxygen.
 Under which reaction conditions would you expect to produce the largest amount of oxygen?
Under which reaction conditions would you expect to produce the largest amount of oxygen?
(Multiple Choice)
4.8/5  (32)
(32)
Magnesium carbonate dissociates to magnesium oxide and carbon dioxide at elevated temperatures.
MgCO3(s)  MgO(s)+ CO2(g)
A reaction vessel contains these compounds in equilibrium at 300°C.What will happen if the volume of the container is reduced by 25% at 300°C?
MgO(s)+ CO2(g)
A reaction vessel contains these compounds in equilibrium at 300°C.What will happen if the volume of the container is reduced by 25% at 300°C?
(Multiple Choice)
4.8/5  (39)
(39)
At 450°C,tert-butyl alcohol decomposes into water and isobutene.
(CH3)3COH(g)  (CH3)2CCH2(g)+ H2O(g)
A reaction vessel contains these compounds at equilibrium.What will happen if the volume of the container is reduced by 50% at constant temperature?
(CH3)2CCH2(g)+ H2O(g)
A reaction vessel contains these compounds at equilibrium.What will happen if the volume of the container is reduced by 50% at constant temperature?
(Multiple Choice)
4.9/5  (42)
(42)
The equilibrium constant,Kp ,for the reaction
CO(g)+ H2O(g)  CO2(g)+ H2(g)
At 986°C is 0.63.A rigid cylinder at that temperature contains 1.2 atm of carbon monoxide,0.20 atm of water vapor,0.30 atm of carbon dioxide,and 0.27 atm of hydrogen.Is the system at equilibrium?
CO2(g)+ H2(g)
At 986°C is 0.63.A rigid cylinder at that temperature contains 1.2 atm of carbon monoxide,0.20 atm of water vapor,0.30 atm of carbon dioxide,and 0.27 atm of hydrogen.Is the system at equilibrium?
(Multiple Choice)
4.8/5  (45)
(45)
Consider the reversible reaction: 2NO2(g)  N2O4(g)
If the concentrations of both NO2 and N2O4 are 0.016 mol L-1,what is the value of Qc?
N2O4(g)
If the concentrations of both NO2 and N2O4 are 0.016 mol L-1,what is the value of Qc?
(Multiple Choice)
4.9/5  (54)
(54)
The equilibrium constant for reaction (1)below is 276.Under the same conditions,what is the equilibrium constant of reaction (2)?
(1) 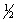 X2(g)+
X2(g)+ 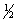 Y2(g)
Y2(g)  XY(g)
(2)2XY(g)
XY(g)
(2)2XY(g)  X2(g)+ Y2(g)
X2(g)+ Y2(g)
(Multiple Choice)
4.7/5  (29)
(29)
Showing 41 - 60 of 85
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)