Exam 4: The Major Classes of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
In a redox reaction, the reducing agent undergoes loss of electrons.
(True/False)
4.8/5  (41)
(41)
A 0.00100 mol sample of Ca(OH)2 requires 25.00 mL of aqueous HCl for neutralization according to the reaction below. What is the concentration of the HCl? Equation: Ca(OH)2(s) + 2HCl(aq) CaCl2(aq) + H2O(l)
(Multiple Choice)
4.9/5  (30)
(30)
In the following reaction, what ions, if any, are spectator ions?
Pb(NO3)2(aq) + 2NaCl(aq) PbCl2(s) + 2NaNO3(aq)
(Multiple Choice)
4.8/5  (40)
(40)
When a solution is diluted with water, the ratio of the initial to final volumes of solution is equal to the ratio of final to initial molarities.
(True/False)
4.9/5  (40)
(40)
You are provided with a 250 mL volumetric flask, deionized water, and solid NaOH. How much NaOH should be weighed out in order to make 250. mL of 0.100 M solution?
(Short Answer)
4.9/5  (42)
(42)
Potassium carbonate, K2CO3, sodium iodide, NaI, magnesium chloride, MgCl2, methanol, CH3OH, and ammonium chloride, NH4Cl, are soluble in water. Which produces the largest number of dissolved particles per mole of dissolved solute?
(Multiple Choice)
4.7/5  (37)
(37)
Which one of the following ionic compounds is insoluble in water?
(Multiple Choice)
4.8/5  (30)
(30)
What, if any, are the spectator ions when aqueous solutions of HBr and RbOH neutralize each other?
(Multiple Choice)
4.7/5  (43)
(43)
A solution of methanol (CH4O) in water has a concentration of 0.200 M. What mass of methanol, in grams, is present in 0.150 liters of this solution?
(Short Answer)
4.8/5  (34)
(34)
Calculate the oxidation number of sulfur in sodium metabisulfite, Na2S2O5.
(Multiple Choice)
4.8/5  (46)
(46)
Select the correct name and chemical formula for the precipitate that forms when the following reactants are mixed. CoSO4(aq) + (NH4)3PO4(aq)
(Multiple Choice)
4.8/5  (34)
(34)
Automobile batteries use 3.0 M H2SO4 as an electrolyte. How much 1.20 M NaOH will be needed to neutralize 225 mL of battery acid?
H2SO4(aq) + 2NaOH(aq) 2H2O(l) + Na2SO4(aq)
(Multiple Choice)
4.9/5  (35)
(35)
How many moles of ions are released when 1.6 mol of ammonium phosphate, (NH4)3PO4, is dissolved in water?
(Multiple Choice)
4.7/5  (33)
(33)
Select the classification for the following reaction.
CaCl2H2O(s) 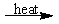 CaCl2(s) + H2O(g)
CaCl2(s) + H2O(g)
(Multiple Choice)
4.8/5  (39)
(39)
Select the classification for the following reaction. Na2O(s) + H2O(l) 2NaOH(aq)
(Multiple Choice)
4.8/5  (32)
(32)
A 0.150 M sodium chloride solution is referred to as a physiological saline solution because it has the same concentration of salts as normal human blood. Calculate the mass of solute needed to prepare 275.0 mL of a physiological saline solution.
(Multiple Choice)
4.9/5  (37)
(37)
In an acid-base (neutralization) reaction the indicator will change color at the end point.
(True/False)
4.8/5  (36)
(36)
Select the classification for the following reaction. H2(g) + Cl2(g) 2HCl(g)
(Multiple Choice)
4.8/5  (45)
(45)
Showing 41 - 60 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)