Exam 4: The Major Classes of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Aqueous potassium iodate (KIO3) and potassium iodide (KI) react in the presence of dilute hydrochloric acid (HCl), as shown below.
KIO3(aq) + 5KI(aq) + 6HCl(aq) 3I2(aq) + 6KCl(aq) + 3H2O(l)
What mass of iodine (I2) is formed when 15.0 mL of 0.0050 M KIO3 solution reacts with 30.0 mL of 0.010 M KI solution in the presence of excess HCl?
(Multiple Choice)
4.9/5  (38)
(38)
Calculate the molarity of a 23.55-mL solution which contains 28.24 mg of sodium sulfate (used in dyeing and printing textiles, 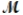 = 139.04 g/mol).
= 139.04 g/mol).
(Multiple Choice)
4.8/5  (36)
(36)
How many moles of H+(aq) ions are present in 750 mL of 0.65 M hydrochloric acid?
(Multiple Choice)
4.9/5  (37)
(37)
A 0.1873 g sample of a pure, solid acid, H2X was dissolved in water and titrated with 0.1052 M NaOH solution. The balanced equation for the neutralization reaction occurring is
H2X(aq) + 2NaOH(aq) Na2X(aq) + 2H2O(l)
If the molar mass of H2X is 85.00 g/mol, calculate the volume of NaOH solution needed in the titration.
(Short Answer)
4.9/5  (34)
(34)
Copper(II) sulfide, CuS, is used in the development of aniline black dye in textile printing. What is the maximum mass of CuS which can be formed when 38.0 mL of 0.500 M CuCl2 are mixed with 42.0 mL of 0.600 M (NH4)2S? Aqueous ammonium chloride is the other product.
(Multiple Choice)
4.9/5  (38)
(38)
Calculate the oxidation number of the chlorine in perchloric acid, HClO4, a strong oxidizing agent.
(Multiple Choice)
4.9/5  (39)
(39)
Aqueous potassium iodate (KIO3) and potassium iodide (KI) react in the presence of dilute hydrochloric acid, as shown below.
KIO3(aq) + 5KI(aq) + 6HCl(aq) 3I2(aq) + 6KCl(aq) + 3H2O(l)
What mass of iodine (I2) is formed when 50.0 mL of 0.020 M KIO3 solution reacts with an excess of KI and HCl?
(Multiple Choice)
4.9/5  (34)
(34)
How many mL of concentrated nitric acid (HNO3, 16.0 M) should be diluted with water in order to make 2.00 L of 2.00 M solution?
(Multiple Choice)
4.8/5  (36)
(36)
Select the classification for the following reaction. Fe(s) + 2Fe3+(aq) 3Fe2+(aq)
(Multiple Choice)
4.8/5  (38)
(38)
Which one of the following ionic compounds is soluble in water?
(Multiple Choice)
4.8/5  (46)
(46)
Chemical reactions generally reach equilibrium because one of the reactants is used up.
(True/False)
5.0/5  (39)
(39)
Calcium chloride is used to melt ice and snow on roads and sidewalks and to remove water from organic liquids. Calculate the molarity of a solution prepared by diluting 165 mL of 0.688 M calcium chloride to 925.0 mL.
(Multiple Choice)
5.0/5  (35)
(35)
Hydrochloric acid is widely used as a laboratory reagent in refining ore for the production of tin and tantalum, and as a catalyst in organic reactions. Calculate the number of moles of HCl in 62.85 mL of 0.453 M hydrochloric acid.
(Multiple Choice)
4.8/5  (36)
(36)
Covalent compounds, dissolved in water, never produce conducting solutions.
(True/False)
4.9/5  (40)
(40)
A standard solution of 0.243 M NaOH was used to determine the concentration of a hydrochloric acid solution. If 46.33 mL of NaOH is needed to neutralize 10.00 mL of the acid, what is the molar concentration of the acid?
(Multiple Choice)
4.7/5  (36)
(36)
Showing 81 - 100 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)