Exam 4: The Major Classes of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Select the classification for the following reaction. 2Ag+(aq) + Zn(s) 2Ag(s) + Zn2+(aq)
(Multiple Choice)
4.9/5  (40)
(40)
Select the classification for the following reaction. 2NaCl(l) 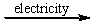 2Na(l) + Cl2(g)
2Na(l) + Cl2(g)
(Multiple Choice)
5.0/5  (46)
(46)
Consider the reaction:
3Co2+(aq) + 6NO3-(aq) + 6Na+(aq) + 2PO43-(aq) Co3(PO4)2(s) + 6Na+(aq) + 6NO3-(aq)
Identify the net ionic equation for this reaction.
(Multiple Choice)
4.8/5  (40)
(40)
For each of the following species, write down, next to the formula, the oxidation number of the indicated atom.
a. P in H2PO2-
b. S in Na2S2O3
c. C in CH2O
(Essay)
4.8/5  (39)
(39)
Which one of the following substances, when dissolved in water at equal molar concentrations, will give the solution with the lowest electrical conductivity?
(Multiple Choice)
4.7/5  (38)
(38)
In a redox reaction, the oxidizing agent undergoes loss of electrons.
(True/False)
4.9/5  (37)
(37)
Which one of the following substances is the best electrolyte?
(Multiple Choice)
4.9/5  (34)
(34)
Select the precipitate that forms when aqueous lead(II) nitrate reacts with aqueous sodium sulfate.
(Multiple Choice)
4.8/5  (36)
(36)
Sulfuric acid (H2SO4) reacts with potassium hydroxide (KOH) as follows.
H2SO4(aq) + 2KOH(aq) K2SO4(aq) + 2H2O(l)
Calculate the volume of 0.100 M sulfuric acid required to neutralize 25.0 mL of 0.0821 M KOH. Show all your work.
(Short Answer)
4.8/5  (36)
(36)
How many milliliters of 1.58 M HCl are needed to react completely with 23.2 g of NaHCO3 ( 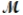 = 84.02 g/mol)? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
= 84.02 g/mol)? HCl(aq) + NaHCO3(s) NaCl(s) + H2O(l) + CO2(g)
(Multiple Choice)
4.8/5  (32)
(32)
Select the net ionic equation for the reaction between sodium chloride and mercury(I) nitrate. 2NaCl(aq) + Hg2(NO3)2(aq) NaNO3(aq) + Hg2Cl2(s)
(Multiple Choice)
4.9/5  (39)
(39)
In each of the following cases, write down the oxidation number of the indicated atom.
a. P in P4
b. C in C2H6
c. S in H2SO4
d. Mn in MnO4-
e. S in S4O62-
f. P in Na3PO4
(Essay)
4.8/5  (30)
(30)
Select the precipitate that forms when the following reactants are mixed. Na2CO3(aq) + BaCl2(aq)
(Multiple Choice)
4.9/5  (32)
(32)
How many moles of ions are released when 0.27 mol of cobalt(II) chloride, CoCl2, is dissolved in water?
(Multiple Choice)
4.7/5  (41)
(41)
Select the classification for the following reaction. 2H2O2(aq) 2H2O(l) + O2(g)
(Multiple Choice)
4.9/5  (42)
(42)
Select the precipitate that forms when the following reactants are mixed.
Mg(CH3COO)2(aq) + LiOH(aq)
(Multiple Choice)
4.8/5  (41)
(41)
The correct method for preparing one liter of a 1.0 M solution of X is to dissolve exactly one mole of X in exactly one liter of water.
(True/False)
4.9/5  (23)
(23)
An aqueous solution of lead nitrate, Pb(NO3)2, is mixed with one of sodium chromate, Na2CrO4, resulting in the formation of a precipitate of lead chromate. Write a balanced net ionic equation for this precipitation reaction, showing all phases.
(Essay)
4.8/5  (52)
(52)
Showing 21 - 40 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)