Exam 4: The Major Classes of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Select the classification for the following reaction. Fe2+(aq) + 2OH-(aq) Fe(OH)2(s)
(Multiple Choice)
4.8/5  (41)
(41)
Predict the products by completing a balanced equation for the following decomposition reaction. CaCl2(l) 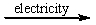 ?
?
(Multiple Choice)
4.8/5  (43)
(43)
Select the classification for the following reaction. H2CO3(aq) H2O(l) + CO2(g)
(Multiple Choice)
4.8/5  (36)
(36)
A particular reaction may be both a precipitation and an acid-base (neutralization) reaction.
(True/False)
4.8/5  (37)
(37)
Write down the oxidation number of the indicated atom in each of the following formulas:
a. Si in SiO2
b. Cl in ClO2-
c. Mn in KMnO4
d. C in C6H12O6
(Essay)
5.0/5  (31)
(31)
How many moles of H+(aq) ions are present in 1.25 L of 0.75 M nitric acid?
(Multiple Choice)
4.8/5  (39)
(39)
Predict the product(s) for the following reaction. BaO(s) + CO2(g)
(Multiple Choice)
5.0/5  (42)
(42)
Which of the statements below correctly describes the combustion of glucose, shown below? C6H12O6 + 6O2 6CO2 + 6H2O
(Multiple Choice)
4.7/5  (36)
(36)
Aluminum metal dissolved in hydrochloric acid as follows:
2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2(g)
a. What is the minimum volume of 6.0 M HCl(aq) needed to completely dissolve 3.20 g of aluminum in this reaction?
b. What mass of AlCl3 would be produced by complete reaction of 3.20 g of aluminum?
(Essay)
4.8/5  (31)
(31)
Magnesium carbonate, MgCO3, is insoluble. Identify the spectator ions when aqueous solutions of sodium carbonate and magnesium chloride are combined.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following solutions will be the best conductor of electrical current?
(Multiple Choice)
4.8/5  (35)
(35)
Select the classification for the following reaction. NH3(aq) + HNO3(aq) NH4NO3(aq)
(Multiple Choice)
4.8/5  (42)
(42)
All acid-base reactions produce a salt and water as the only products.
(True/False)
4.8/5  (31)
(31)
Sodium chlorate is used as an oxidizer in the manufacture of dyes, explosives, and matches. Calculate the mass of solute needed to prepare 1.575 L of 0.00250 M NaClO3 ( 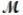 = 106.45 g/mol).
= 106.45 g/mol).
(Multiple Choice)
4.9/5  (43)
(43)
Select the best statement relating to the following reaction:
2MnO2(s) + KClO3(aq) + 2KOH(aq) 2KMnO4(aq) + KCl(aq) + H2O(l)
(Multiple Choice)
4.7/5  (36)
(36)
Complete and balance the equation for the following acid-base reaction.
Ca(OH)2(aq) + HCl(aq)
(Essay)
4.9/5  (35)
(35)
Showing 61 - 80 of 124
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)