Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
Consider the reaction
11ec6d53_97e1_49fd_9cd1_030bf8888a28_TB5833_
If the concentrations of the Cu+ and I- ions in equilibrium at 298 K are both equal to 1.03 × 10-6 M, what is the value of G° for the reaction?
(Multiple Choice)
5.0/5  (38)
(38)
In the expression, S = k ln W, W is called the number of microstates. Explain clearly the meaning of the word "microstate", and why a system under a given set of conditions normally has many microstates.
(Essay)
4.8/5  (35)
(35)
Which of the following is true for pure oxygen gas, O2(g) at 25°C?
(Multiple Choice)
4.8/5  (35)
(35)
Which relationship best describes S° for the following reaction? 8H2(g) + S8(s) 8H2S(g)
(Multiple Choice)
4.9/5  (29)
(29)
The reaction of methane with water to form carbon dioxide and hydrogen is non-spontaneous at 298 K. At what temperature will this system make the transition from non-spontaneous to spontaneous? The data refer to 298 K.
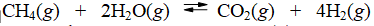
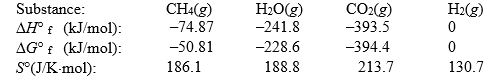
(Multiple Choice)
4.9/5  (39)
(39)
Which relationship or statement best describes S° for the following reaction? BaCl2(aq) + Na2SO4(aq) BaSO4(s) + 2NaCl(aq)
(Multiple Choice)
4.8/5  (40)
(40)
Consider the figure which shows G° for a chemical process plotted against absolute temperature. From this plot, it is reasonable to conclude that: 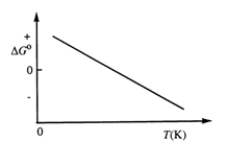
(Multiple Choice)
4.8/5  (33)
(33)
For what signs of H and S will a process
a. be spontaneous at high temperatures but not at low temperatures?
b. not be spontaneous at any temperatures?
(Essay)
4.8/5  (31)
(31)
Use the given data at 298 K to calculate G° for the reaction 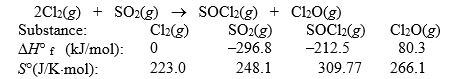
(Multiple Choice)
4.7/5  (40)
(40)
Which relationship or statement best describes S° for the following reaction?
2NH3(g) + 2ClF3(g) 6HF(g) + N2(g) + Cl2(g)
(Multiple Choice)
4.8/5  (43)
(43)
Elemental boron can be formed by reaction of boron trichloride with hydrogen. 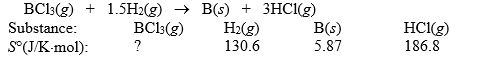 If S° = 80.3 J/K for the reaction above, what is S° for BCl3(g)?
If S° = 80.3 J/K for the reaction above, what is S° for BCl3(g)?
(Multiple Choice)
4.9/5  (34)
(34)
Consider the figure which shows G° for a chemical process plotted against absolute temperature. From this plot, it is reasonable to conclude that: 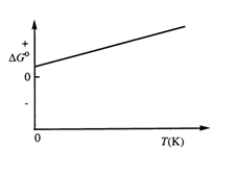
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following is necessary for a process to be spontaneous?
(Multiple Choice)
4.8/5  (46)
(46)
Sulfuryl dichloride is formed when sulfur dioxide reacts with chlorine. The data refer to 298 K. SO2(g) + Cl2(g) SO2Cl2(g) 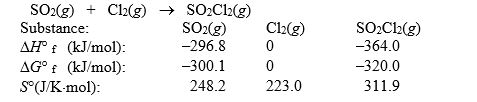 What is the value of G° for this reaction at 600 K?
What is the value of G° for this reaction at 600 K?
(Multiple Choice)
4.9/5  (22)
(22)
The higher the pressure of a gas sample, the greater is its entropy.
(True/False)
4.8/5  (39)
(39)
In which one of these pairs will the entropy of the first substance be greater than that of the second? Assume P and T are the same for each pair, unless stated otherwise.
(Multiple Choice)
4.8/5  (31)
(31)
Given: H2O(l) H2O(s) H° = -6.02 kJ at 273K Calculate the entropy change of the surroundings (Ssurr) when one mole of water freezes at 0 °C and a pressure of one atmosphere.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 41 - 60 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)