Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
For a chemical reaction to be spontaneous at all temperatures, which of the following conditions must be met?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following is true for a system at equilibrium?
(Multiple Choice)
4.7/5  (36)
(36)
As a chemical reaction proceeds toward equilibrium, the free energy of the system decreases.
(True/False)
4.8/5  (36)
(36)
Given: C2H2(g) 2C(graphite) + H2(g) G° = -209 kJ
A sample of gaseous C2H2 (acetylene, or ethyne) was stored for one year, yet at the end of this period the sample remained unchanged and no graphite or hydrogen gas had been formed. Briefly explain why there is no inconsistency between the sign of G° and the apparent stability of the sample.
(Essay)
4.8/5  (43)
(43)
Nitric oxide reacts with chlorine to form NOCl. The data refer to 298 K. 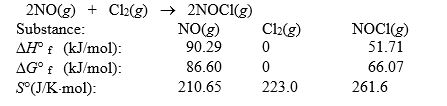 What is the value of G° for this reaction at 550 K?
What is the value of G° for this reaction at 550 K?
(Multiple Choice)
4.9/5  (34)
(34)
A reaction has a positive value of H° and a positive value of S°.
Draw a neat, labeled schematic plot to show how G° (y-axis) will depend on absolute temperature (x-axis).
(Essay)
4.8/5  (39)
(39)
Which relationship or statement best describes S° for the following reaction?
KCl(s) K+(aq) + Cl-(aq)
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following is always true for an endothermic process?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following should have the greatest molar entropy at 298 K?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following values is based on the Third Law of Thermodynamics?
(Multiple Choice)
4.8/5  (35)
(35)
Which, if any, of the following processes is spontaneous under the specified conditions?
(Multiple Choice)
4.9/5  (40)
(40)
You are given pure samples of ammonia, NH3(g), and nitrogen trifluoride, NF3(g). What prediction would you make concerning their standard molar entropies at 298 K?
(Multiple Choice)
4.8/5  (47)
(47)
Showing 81 - 94 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)