Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions
Exam 1: Keys to the Study of Chemistry79 Questions
Exam 2: The Components of Matter105 Questions
Exam 3: Stoichiometry of Formulas and Equations87 Questions
Exam 4: The Major Classes of Chemical Reactions124 Questions
Exam 5: Gases and the Kinetic-Molecular Theory115 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change85 Questions
Exam 7: Quantum Theory and Atomic Structure83 Questions
Exam 8: Electron Configuration and Chemical Periodicity85 Questions
Exam 9: Models of Chemical Bonding74 Questions
Exam 10: The Shapes of Molecules109 Questions
Exam 11: Theories of Covalent Bonding58 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes111 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids105 Questions
Exam 14: Periodic Patterns in the Main Group Elements: Bonding, Structure, and Reactivity118 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon118 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions88 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions104 Questions
Exam 18: Acid-Base Equilibria103 Questions
Exam 19: Ionic Equilibria in Aqueous Systems119 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions94 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry57 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications94 Questions
Select questions type
When a sky diver free-falls through the air, the process is
(Multiple Choice)
4.9/5  (40)
(40)
A certain process has Suniv > 0 at 25°C. What does one know about the process?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following results in a decrease in the entropy of the system?
(Multiple Choice)
4.8/5  (49)
(49)
Which of the following pairs has the member with the greater molar entropy listed first? All systems are at 25°C.
(Multiple Choice)
4.8/5  (32)
(32)
"A diamond is forever" is one of the most successful advertising slogans of all time. But is it true? For the reaction shown below, calculate the standard free energy change at 298K and determine whether or not a diamond is "forever". C(diamond) C(graphite)
Data: Hf°(diamond) = 1.895 kJ/mol; S°(diamond) = 2.337 J mol-1 K-1;
S°(graphite) = 5.740 J mol-1K-1.
(Multiple Choice)
4.9/5  (43)
(43)
Calculate the equilibrium constant at 25°C for the reaction of methane with water to form carbon dioxide and hydrogen. The data refer to
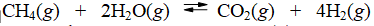
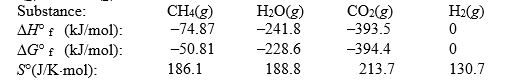
(Multiple Choice)
4.8/5  (40)
(40)
Which relationship or statement best describes S° for the following reaction?
O3(g) + NO(g) O2(g) + NO2(g)
(Multiple Choice)
4.7/5  (30)
(30)
For a process with S < 0, which one of the following statements is correct?
(Multiple Choice)
4.8/5  (31)
(31)
Which relationship or statement best describes S° for the following reaction?
2H2S(g) + 3O2(g) 2H2O(g) + 2SO2(g)
(Multiple Choice)
4.8/5  (41)
(41)
Consider the following quantities used in thermodynamics: E, H, q, w, S, G. How many of them are state functions?
(Multiple Choice)
4.9/5  (38)
(38)
The term microstate refers to the energy state of a single molecule in a system of many molecules.
(True/False)
4.9/5  (39)
(39)
In some spontaneous processes, the entropy of the surroundings decreases.
(True/False)
4.8/5  (36)
(36)
For the reaction of xenon and fluorine gases to form solid XeF4, H° = -251 kJ and G° = -121 kJ at 25°C. Calculate S° for the reaction.
(Short Answer)
4.9/5  (36)
(36)
In which one of the following pairs will the first system have a higher entropy than the second? Assume P and T are the same for each pair, unless stated otherwise.
(Multiple Choice)
4.9/5  (34)
(34)
A reaction is proceeding toward equilibrium. At a certain stage, the concentrations of reactants and products are such that G = G°. What conclusion can reasonably be drawn about the reaction at this time?
(Multiple Choice)
4.9/5  (41)
(41)
In a spontaneous process, the entropy of the system always increases.
(True/False)
4.8/5  (37)
(37)
Which of the following is always true for an exothermic process?
(Multiple Choice)
4.8/5  (33)
(33)
Which one of the following phase changes decreases the entropy of the system?
(Multiple Choice)
4.8/5  (31)
(31)
The entropy of one mole of oxygen gas in a 0.5-L container is less than it would be in a 22.4-L container at the same temperature.
(True/False)
4.8/5  (41)
(41)
Showing 61 - 80 of 94
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)