Exam 3: Stoichiometry: Mass, Formulas, and Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Elemental analysis of the soot produced by a candle flame shows that it is 14.3% H and 85.7% C by mass. What is the empirical formula of this hydrocarbon?
(Multiple Choice)
4.9/5  (30)
(30)
Nicotine is an alkaloid and a component of cigarettes that contribute to the addictive properties of tobacco. Mass analysis reveals that it has the empirical formula C5H7N. If the molar mass is 162.23 g/mol, which molecular formula is correct?
(Multiple Choice)
4.8/5  (43)
(43)
Identify the set of stoichiometric coefficients that balances the reaction equation for the combustion of octane. C8H18 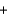 O2
O2  CO2
CO2  H2O
H2O
(Multiple Choice)
4.9/5  (30)
(30)
Fuming sulfuric acid is obtained by the addition of SO3 to concentrated H2SO4. The fumes result from the reaction of SO3 gas with water vapor. What is the product when one molecule of SO3 reacts with one molecule of water?
(Multiple Choice)
4.7/5  (35)
(35)
A gas is produced when fruit ripens. That gas can be used in mixtures with nitrogen gas to ripen bananas just before they are put on the grocery shelf. This mixture is sold as banana gas. The mass percentages of carbon and hydrogen in this gas are 85.62% for C and 14.38% for H. The molar mass was found to be twice the empirical formula unit molar mass. What is the molecular formula of this gas?
(Multiple Choice)
4.8/5  (37)
(37)
Which of the following oxides of nitrogen have the same empirical formulas?
N2O, NO, NO2, N2O2, N2O4
(Essay)
4.7/5  (45)
(45)
Calculate the mass percent of each element in Ibuprofen (C13H18O2), a drug typically used to treat pain, fever, or inflammation.
(Short Answer)
4.8/5  (42)
(42)
A sample of water (H2O) contains 1.81  1024 molecules. How many total moles of atoms are there in this sample?
1024 molecules. How many total moles of atoms are there in this sample?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following contains the largest number of atoms?
(Multiple Choice)
5.0/5  (29)
(29)
One form of asbestos called chrysotile is considered to be a human carcinogen. Mass analysis reveals that it has the empirical formula Mg3Si2H4O9. If the molar mass is 831 g/mol, which molecular formula is correct?
(Multiple Choice)
4.8/5  (32)
(32)
At the Tesla automotive factory, a Model S sedan is built using 4 tires, a main chassis, and 5 lithium ion batteries. How many sedans can be made if there are 17 tires, 5 chassis, and 22 batteries?
(Multiple Choice)
4.8/5  (41)
(41)
A reaction vessel contains equal masses of iron and oxygen. How much FeO could theoretically be produced?
(Multiple Choice)
4.8/5  (35)
(35)
Which molecules shown below have the same molecular formula? 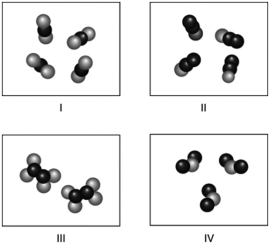
(Multiple Choice)
4.8/5  (28)
(28)
For which of the following compounds are the empirical and molecular formulas the same?
Acetic acid, found in vinegar, CH3COOH
Formaldehyde, used to preserve biological specimens, CH2O
Ethanol, found in beer and wine, CH3CH2OH
(Short Answer)
4.7/5  (32)
(32)
Copper sulfate is a blue solid that is used to control algae growth. Solutions of copper sulfate that come in contact with the surface of galvanized (zinc-plated) steel pails undergo the following reaction that forms copper metal on the zinc surface. How many grams of zinc would react with 454 g (1 lb) of copper sulfate (160 g/mol)? CuSO4(aq) 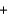 Zn(s)
Zn(s)  Cu(s)
Cu(s) 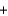 ZnSO4(aq)
ZnSO4(aq)
(Multiple Choice)
4.8/5  (35)
(35)
What mass of phosphoric acid (H3PO4, 98.0 g/mol) is produced from the reaction of 10.0 g of P4O10 (284 g/mol) with excess water?
(Multiple Choice)
4.8/5  (24)
(24)
As a purchasing agent for a pharmaceutical company, how much chlorine, Cl2, do you need to order to react completely with 500 kg of platinum, Pt, to make cisplatin, PtCl2(NH3)2?
(Multiple Choice)
4.8/5  (34)
(34)
Which statement about the following chemical reaction is not correct? 2NH3 + 2O2 N2O + 3H2O
(Multiple Choice)
4.8/5  (40)
(40)
Showing 41 - 60 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)