Exam 3: Stoichiometry: Mass, Formulas, and Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
The empirical formula for buckminsterfullerene is C1, and its molar mass is 720.6 g/mol. What is its molecular formula?
(Multiple Choice)
5.0/5  (36)
(36)
Caffeine has an elemental analysis of 49.48% carbon, 5.190% hydrogen, 16.47% oxygen, and 28.85% nitrogen. It has a molar mass of 194.19 g/mol. What is the molecular formula of caffeine?
(Multiple Choice)
4.9/5  (30)
(30)
How many nitrogen atoms are there in 51.0 g of (NH4)2SO4 (ammonium sulfate)?
(Multiple Choice)
4.8/5  (31)
(31)
Phosphorus trichloride reacts with water to form phosphorous acid, H3PO3, and hydrochloric acid in the following unbalanced reaction. For each mole of phosphorous acid produced by this reaction, how many moles of HCl are produced?__ PCl3 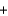 __H2O
__H2O  __H3PO3
__H3PO3 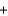 + __ HCl
+ __ HCl
(Multiple Choice)
4.7/5  (35)
(35)
Identify the list below that has these chloride compounds arranged in order of increasing molar mass.
(Multiple Choice)
4.9/5  (42)
(42)
Mesitylene is a liquid hydrocarbon. If 0.115 g of this compound is burned in pure oxygen to give 0.3790 g CO2 and 0.1035 g H2O, what is the empirical formula of the compound?
(Short Answer)
5.0/5  (37)
(37)
The combustion of heptane (C7H16) forms carbon dioxide and water. What is the stoichiometric coefficient for water in the balanced equation when 1 mol of heptane undergoes combustion?
(Multiple Choice)
4.7/5  (46)
(46)
A copper ore consisting of 12.5% copper(II) sulfide, when heated and reacted with oxygen gas, produces copper(II) oxide and sulfur dioxide. If the reaction has a 90.0% yield, how many grams of copper(II) oxide are produced when 1.00 kg of the copper ore is processed?
(Multiple Choice)
4.9/5  (36)
(36)
A range of organic molecules can undergo combustion. If pyridine (C5H5N) undergoes combustion through the following balanced chemical reaction, how much carbon dioxide can be produced when 3.2 g of pyridine reacts? 4C5H5N 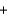 27O2
27O2
 H2O
H2O 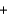 20CO2
20CO2 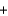 4NO
4NO
(Multiple Choice)
4.8/5  (36)
(36)
Metallic sodium was originally produced by the Deville process, where sodium carbonate and carbon black are heated to 1100 C to produce sodium (as a liquid at that temperature) and carbon monoxide gas, according to the following balanced equation.
Na2CO3(s ) 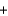 2C(s )
2C(s )  2Na(l)
2Na(l) 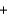 3CO(g)
If 2.3 kg of sodium carbonate is heated, what is the maximum amount of sodium that can be produced?
3CO(g)
If 2.3 kg of sodium carbonate is heated, what is the maximum amount of sodium that can be produced?
(Short Answer)
4.8/5  (35)
(35)
In which of the following reaction mixtures, where A reacts with B in the following balanced equation, is B the limiting reactant? A 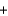 2B
2B  AB2
AB2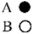
(Multiple Choice)
5.0/5  (30)
(30)
Which of the following cartoons depict a balanced reaction?
(Multiple Choice)
4.9/5  (37)
(37)
Copper was the first metal to be produced from its ore because it is the easiest to smelt, that is, to refine by heating in the presence of carbon (hence the early occurrence of the Bronze Age). The ore was likely malachite (Cu2(OH)2CO3). What is the mass percent of copper in malachite?
(Multiple Choice)
4.8/5  (40)
(40)
Which of the following molecules have identical molecular and empirical formulas? 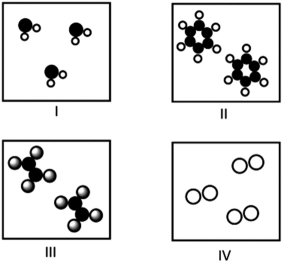
(Multiple Choice)
4.8/5  (41)
(41)
Chemical analysis of an organic compound found the following composition: 40.0% C, 53.5% O, and 6.7% H. If the molar mass is 180.2 g/mol, how many empirical formula units are there in the molecular formula?
(Multiple Choice)
4.9/5  (45)
(45)
Which statement A-D regarding photosynthesis is not correct? In the process of photosynthesis ________
(Multiple Choice)
4.9/5  (31)
(31)
Ammonia, NH3, is an important industrial chemical. It is produced using the Haber process from nitrogen, N2, and hydrogen, H2. How much ammonia can be produced from 28 kg of nitrogen and 2 kg of hydrogen, assuming the reaction yield is 90%?
(Multiple Choice)
4.7/5  (39)
(39)
Showing 121 - 140 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)