Exam 3: Stoichiometry: Mass, Formulas, and Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
How many chlorine atoms are there in 16.0 g of carbon tetrachloride (CCl4)?
(Multiple Choice)
4.8/5  (45)
(45)
A tiny speck (8.3 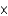 10-7 mol) of radioactive americium-241 is used in smoke detectors. How many atoms of americium-241 are there in one of these smoke detectors?
10-7 mol) of radioactive americium-241 is used in smoke detectors. How many atoms of americium-241 are there in one of these smoke detectors?
(Multiple Choice)
4.9/5  (34)
(34)
Write a definition of the phrase "stoichiometry of a chemical reaction."
(Essay)
4.9/5  (39)
(39)
Which substance listed below contains the most oxygen atoms?
(Multiple Choice)
4.8/5  (42)
(42)
What is the formula mass of (NH4)3PO4 (ammonium phosphate)?
(Multiple Choice)
4.7/5  (29)
(29)
Which molecules shown below have the same empirical formula? 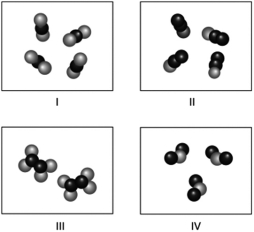
(Multiple Choice)
4.8/5  (33)
(33)
One form of asbestos called chrysotile is considered to be a human carcinogen. Mass analysis shows that the composition of chrysotile is 26.3% Mg, 20.2% Si, 1.45% H, and the remainder of the mass is oxygen. Determine the empirical formula of chrysotile.
(Multiple Choice)
4.9/5  (37)
(37)
Carbon monoxide is used in refining iron ore to produce the metal. Assuming the reaction yield is 100%, how many grams of iron would be produced if 5.00 mol of carbon monoxide were exposed to 800 g iron(III) oxide?
Fe2O3(s) 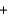 3CO(g)
3CO(g)  2Fe(s)
2Fe(s) 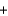 3CO2(g)
3CO2(g)
(Short Answer)
4.8/5  (35)
(35)
Chlorhexidine is an antibacterial drug found in mouthwash and has an elemental analysis of 52.28% carbon, 5.98% hydrogen, 14.03% chlorine, and 27.71% nitrogen. It has a molar mass of 505.45 g/mol. What is the molecular formula of chlorhexidine?
(Multiple Choice)
5.0/5  (31)
(31)
Ozone (O3) reacts with iodide (I-) and water to form iodine (I2), hydroxide (OH-), and oxygen (O2). Balance the following reaction equation and report the sum of the stoichiometric coefficients. O3 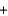 I-
I-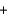 H2O
H2O  I2
I2 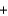 OH-
OH-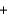 O2
O2
(Multiple Choice)
4.8/5  (29)
(29)
Combustion analysis of an organic compound to determine the percentages of carbon, hydrogen, and oxygen in the compound depends on which of the following assumptions?
(Multiple Choice)
4.9/5  (38)
(38)
How many oxygen atoms are there in 27.0 g of sodium sulfate (Na2SO4)?
(Multiple Choice)
4.9/5  (43)
(43)
Elemental analysis of the organic compound meta-xylene shows that it is 9.49% H and 90.51% C by mass. What is the empirical formula of this hydrocarbon?
(Multiple Choice)
4.9/5  (37)
(37)
Which statement A-D regarding the terms mole and molar mass is not correct?
(Multiple Choice)
4.8/5  (37)
(37)
Air bags in cars inflate when an electrical spark activates sodium azide (NaN3) so that it decomposes to sodium metal, Na, and nitrogen gas, N2. In the balanced reaction equation, how many moles of nitrogen gas are formed for each mole of sodium azide?
(Multiple Choice)
4.9/5  (31)
(31)
Allicin, a potent antibacterial compound, is formed by the enzyme alliinase when garlic is chopped or damaged. Allicin contains carbon, hydrogen, oxygen, and sulfur atoms. In combustion analysis, a 10.00 mg sample of allicin produced 16.27 mg of CO2, 5.55 mg of water, and 7.90 mg of SO2. What is the empirical formula of allicin?
(Multiple Choice)
4.8/5  (34)
(34)
What is the difference in the chemistry between photosynthesis and respiration?
(Essay)
4.8/5  (32)
(32)
How many moles of ammonia are there in a 346 g sample of pure NH3 (17.03 g/mol)?
(Multiple Choice)
4.8/5  (39)
(39)
The combustion of fossil fuels is the main anthropogenic source of carbon dioxide in the atmosphere. During the combustion of gasoline in automobile engines, oxygen reacts with hydrocarbons to produce carbon dioxide and water. Assume gasoline is octane (C8H18). Write the balanced reaction equation, and report the sum of the stoichiometric coefficients written as integers.
(Multiple Choice)
4.8/5  (38)
(38)
Showing 101 - 120 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)