Exam 3: Stoichiometry: Mass, Formulas, and Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
One form of elemental sulfur is a ring of eight sulfur atoms. How many moles of molecular oxygen are consumed when one mole of this allotrope burns to make sulfur trioxide?
(Multiple Choice)
4.8/5  (35)
(35)
A reaction vessel contains equal masses of solid magnesium metal and oxygen gas. The mixture is ignited and burns with a burst of light and heat, producing solid MgO. The mass of the MgO is less than the initial mass of the magnesium and oxygen. What is your explanation for this apparent loss of mass?
(Multiple Choice)
4.8/5  (36)
(36)
How many atoms of chlorine are there in 25.0 g of calcium chloride (CaCl2)?
(Multiple Choice)
5.0/5  (34)
(34)
A bottle containing 1,665 g of sulfuric acid (H2SO4, 98.08 g/mol) was spilled in a laboratory. The emergency spill kit contained a full 2.0 kg bottle of sodium carbonate (105.99 g/mol). Is this enough sodium carbonate to neutralize the acid, according to the following reaction? H2SO4(aq) 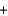 Na2CO3(s)
Na2CO3(s)  Na2SO4(aq)
Na2SO4(aq) 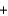 CO2(g)
CO2(g) 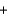 H2O(l)
H2O(l)
(Multiple Choice)
4.8/5  (37)
(37)
Write the balanced reaction equation for the combustion of nonane (C9H20), which is found in gasoline.
(Essay)
4.9/5  (39)
(39)
Some indoor air purification systems work by converting some of the oxygen in the air to ozone. The ozone oxidizes mold, mildew spores, and other biological pollutants. It is claimed that one such system generates 5.0 g of ozone per hour by passing dry air through the purifier at a rate of 5.0 L/min. If 1.0 L of air contains 0.28 g of oxygen, what percent of the O2 molecules are converted to O3 molecules?
(Short Answer)
4.7/5  (33)
(33)
You are given a liquid sample that contains either methanol (CH3OH), ethanol (CH3CH2OH), or a mixture of both. You use combustion analysis of a 0.336 g sample and obtain 0.462 g of CO2. What did you learn about your sample?
(Multiple Choice)
4.9/5  (23)
(23)
For a standard airbag, 131 g of sodium azide (65.00 g/mol) is required to generate enough gas to adequately fill the airbag. How many formula units of sodium azide are needed?
(Multiple Choice)
4.9/5  (35)
(35)
Which statement about the combustion of propane (C3H8) is not correct? C3H8
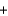 5O2
5O2
 3CO2
3CO2
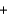 4H2O
4H2O
(Multiple Choice)
4.8/5  (32)
(32)
Glucose (C6H12O6) is oxidized by molecular oxygen to carbon dioxide and water. How many O2 molecules are needed for each molecule of glucose that is oxidized?
(Multiple Choice)
4.9/5  (33)
(33)
Calcium hydride (CaH2) is so reactive with water that it can be used to remove traces of water from solvents other than water. If 24.6 g of calcium hydride is added to a large volume of solvent that contains 14.0 g of water, which of these remains, water or calcium hydride, after the reaction is complete? The reaction is: CaH2(s) 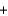 2H2O(l)
2H2O(l)  Ca(OH)2(s)
Ca(OH)2(s) 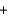 2H2(g)
2H2(g)
(Multiple Choice)
4.9/5  (32)
(32)
A mass of 11.60 g of phosphoric acid was produced from the reaction of 10.00 g of P4O10 with 12.00 g water. What was the percent yield for this reaction?
(Multiple Choice)
4.8/5  (35)
(35)
Sulfur dioxide from coal-fired power plants combines with water in the atmosphere to produce acid rain. What is the product when one molecule of SO2 reacts with one molecule of water?
(Multiple Choice)
4.8/5  (39)
(39)
Water can be separated into its elements according to the following equation by electrolysis. If 20.0 g of water is decomposed by this method, how much oxygen gas is produced? 2H2O(l)  2H2(g)
2H2(g) 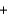 O2(g)
O2(g)
(Multiple Choice)
4.8/5  (29)
(29)
The acid-base reaction between phosphoric acid, H3PO4, and calcium hydroxide, Ca(OH)2, yields water and calcium phosphate. For each mole of calcium phosphate produced by this reaction, how many moles of water are produced?
(Multiple Choice)
4.7/5  (36)
(36)
Beryllium metal reacts with chlorine gas to produce beryllium chloride (BeCl2). Given the following diagram of available beryllium atoms and chlorine molecules in a reaction flask, identify the excess reagent and how many atoms/molecules of reactant remain at the end of the reaction. 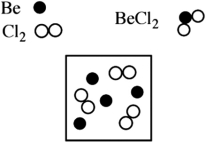
(Multiple Choice)
4.9/5  (33)
(33)
U.S. Lime & Minerals is a company that produces quicklime, CaO, by heating calcium hydroxide, Ca(OH)2. What is the percent yield if 45 grams of quicklime are obtained from 1 mole of calcium hydroxide?
(Multiple Choice)
4.9/5  (41)
(41)
Fool's Gold is the mineral pyrite (FeS2). What is the mass percent of sulfur in pyrite?
(Multiple Choice)
4.9/5  (40)
(40)
Which one of the following samples contains the largest number of molecules?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 81 - 100 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)