Exam 3: Stoichiometry: Mass, Formulas, and Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
Which of the following cartoons depict a balanced reaction?
(Multiple Choice)
4.8/5  (26)
(26)
Nitrogen monoxide undergoes combustion to produce nitrogen dioxide. Which relationship regarding the quantities of reactants and products associated with this reaction is not correct? 2NO 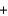 O2
O2  2NO2
2NO2
(Multiple Choice)
4.8/5  (34)
(34)
Without doing any calculations, identify which of the following compounds has the largest percent sodium by mass and which has the smallest. Explain your logic.
sodium oxide, Na2O; sodium hydroxide, NaOH; sodium hydrogen carbonate, NaHCO3;
sodium carbonate, Na2CO3; sodium peroxide, Na2O2
(Essay)
4.9/5  (40)
(40)
At Sal's Sandwich Shop, a regular club sub is made using 2 slices of bread, 3 pieces of meat, and 1 cheese slice. How many club sandwiches can be made if there are 7 slices of bread, 10 pieces of meat, and 5 cheese slices?
(Multiple Choice)
4.9/5  (31)
(31)
When are an empirical formula and a molecular formula the same?
(Multiple Choice)
4.7/5  (37)
(37)
A compound known to contain platinum, nitrogen, and hydrogen was analyzed and found to contain 74.1% Pt and 21.3% N; the remainder was hydrogen. What is the empirical formula for the compound?
(Multiple Choice)
4.9/5  (37)
(37)
The average car emits 8.0 kg of carbon dioxide per gallon of combusted gas. What mass of gasoline (C8H18, 114.23 g/mol) was required to make that much carbon dioxide?
(Multiple Choice)
4.8/5  (37)
(37)
Phosphorus pentoxide is a common drying agent of organic molecules. Mass analysis reveals that it has the empirical formula P2O5. If the molar mass is 283.89 g/mol, which molecular formula is correct?
(Multiple Choice)
4.8/5  (33)
(33)
List three reasons why the actual yield for a chemical reaction may differ from the theoretical yield.
(Essay)
4.7/5  (39)
(39)
In one analysis, 1.34 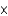 1017 molecules of ozone (O3) were found in 2.00 mL of an air sample. How many moles of oxygen atoms is this?
1017 molecules of ozone (O3) were found in 2.00 mL of an air sample. How many moles of oxygen atoms is this?
(Multiple Choice)
4.9/5  (39)
(39)
Which statement about the following chemical reaction is not correct? 3H2 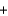 N2
N2
 2NH3
2NH3
(Multiple Choice)
4.9/5  (35)
(35)
Dialuminum hexachloride, Al2Cl6 (266.66 g/mol), is an inexpensive compound that is used in many industrial processes. It is made by treating scrap aluminum with chlorine gas. If a reaction is run with 270 g of aluminum and 710 grams of chlorine, the limiting reactant is ________, and the theoretical yield is ________ of Al2Cl6. 2Al(s) 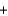 3Cl2(g)
3Cl2(g)  Al2Cl6(s)
Al2Cl6(s)
(Multiple Choice)
4.8/5  (25)
(25)
Fe2O3(s) and powdered aluminum can react with great output of heat to form molten iron and Al2O3. When this reaction equation is balanced, what are the stoichiometric coefficients in the following order: Fe2O3, Al, Fe, Al2O3?
(Multiple Choice)
4.9/5  (30)
(30)
Even though lead is toxic, lead compounds were used in ancient times as white pigments in cosmetics. What is the percentage of lead by mass in lead(IV) carbonate, Pb(CO3)2?
(Multiple Choice)
4.8/5  (35)
(35)
A burner in a gas grill mixes 20 volumes of air for every one volume of propane (C3H8). For gases at a given temperature and pressure, the volume that the gas occupies is directly proportional to the number of moles of the gas. Air is 21% oxygen by volume. Is the mixture produced by this burner rich (excess propane), lean (excess oxygen), or just right (a stoichiometric mixture of propane and oxygen)?
(Essay)
4.8/5  (37)
(37)
How many hydrogen atoms are there in a 346 g sample of pure ammonia (NH3)?
(Multiple Choice)
4.9/5  (35)
(35)
Diesel fuel for automobiles and trucks is a mixture of hydrocarbons that can be modeled by C16H34. Write the balanced reaction equation for the combustion of C16H34, and report the sum of the stoichiometric coefficients.
(Multiple Choice)
4.8/5  (28)
(28)
Phthalocyanine is a large molecule used in printing inks and dyes for clothing due to its insolubility in most solvents, its chemical stability, and its intense blue color. Elemental analysis showed that it consists of 74.69% C, 3.525% H, and 21.77% N, and has a molar mass of 514.54 g/mol. What is the molecular formula for phthalocyanine?
(Multiple Choice)
4.9/5  (27)
(27)
A particular manufacturer sells phosphoric acid capsules as a homeopathic remedy with a concentration specified as 23X. (23X means that when averaged over many capsules, the average mass of phosphoric acid in one capsule is 10-23 times the mass of the capsule. The rest is a carbohydrate filler.) If each capsule has a mass of 35 mg, approximately how many capsules would you have to take to expect to ingest at least one molecule of H3PO4 (98.0 g/mol)?
(Multiple Choice)
4.8/5  (45)
(45)
Showing 21 - 40 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)