Exam 3: Stoichiometry: Mass, Formulas, and Reactions
Exam 1: Matter and Energy: The Origin of the Universe103 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here144 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions141 Questions
Exam 4: Solution Chemistry: The Hydrosphere148 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions139 Questions
Exam 6: Properties of Gases: The Air We Breathe164 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles166 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas156 Questions
Exam 9: Molecular Geometry: Shape Determines Function188 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water165 Questions
Exam 11: Solutions: Properties and Behavior150 Questions
Exam 12: Solids: Crystals, Alloys, and Polymers130 Questions
Exam 13: Chemical Kinetics: Reactions in the Atmosphere155 Questions
Exam 14: Chemical Equilibrium: How Much Product Does a Reaction Really Make111 Questions
Exam 15: Acidbase Equilibria: Proton Transfer in Biological Systems122 Questions
Exam 16: Additional Aqueous Equilibria: Chemistry and the Oceans127 Questions
Exam 17: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes186 Questions
Exam 18: Electrochemistry: The Quest for Clean Energy167 Questions
Exam 19: Nuclear Chemistry: Applications to Energy and Medicine165 Questions
Exam 20: Organic and Biological Molecules: the Compounds of Life168 Questions
Exam 21: The Main Group Elements: Life and the Periodic Table96 Questions
Exam 22: Transition Metals: Biological and Medical Applications142 Questions
Select questions type
The combustion of ethanol (CH3CH2OH, 46.1 g/mol) results in the formation of water and carbon dioxide. How many grams of carbon dioxide are produced when 46.1 g of ethanol burns?
(Multiple Choice)
4.8/5  (35)
(35)
Which statement about a balanced chemical reaction equation is always correct?
(Multiple Choice)
4.9/5  (40)
(40)
A compound was analyzed and found to contain 25.24% S and 74.76% F. What is the empirical formula for the molecular compound?
(Multiple Choice)
4.8/5  (31)
(31)
Baking soda (NaHCO3, 84.0 g/mol) requires acids from other ingredients to generate the carbon dioxide needed to make bread rise. The following equation describes this reaction, where HB is some unspecified acid. If 20.4 g of baking soda are used in a recipe and enough acid is present for a complete reaction, how many moles of carbon dioxide are generated? HB 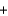 NaHCO3
NaHCO3  H2O
H2O 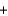 CO2
CO2 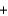 NaB
NaB
(Multiple Choice)
4.7/5  (26)
(26)
In one analysis, the density of ozone in an air sample was found to be 5.00 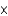 10-8 mol/L. What is the density in molecules per liter?
10-8 mol/L. What is the density in molecules per liter?
(Multiple Choice)
4.9/5  (30)
(30)
Write the balanced reaction equation for the decomposition of ammonium nitrate into nitrogen gas, oxygen gas, and water vapor.
(Essay)
4.9/5  (40)
(40)
Diborane undergoes combustion according to the following unbalanced reaction.
B2H6 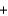 O2
O2  HBO2
HBO2 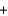 H2O
Balance the equation and calculate how much HBO2 can be formed from 12.6 g of B2H6?
H2O
Balance the equation and calculate how much HBO2 can be formed from 12.6 g of B2H6?
(Essay)
4.8/5  (32)
(32)
Which statement about the following chemical reaction is not correct? 2H2
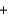 O2
O2
 2H2
2H2
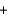 O2
O2
(Multiple Choice)
4.7/5  (51)
(51)
The most abundant metal in Earth's crust is aluminum, found mostly in the form of clays. There are no economical routes for extracting aluminum from clay. However, bauxite ore, impure hydrated aluminum oxide, is found in hot, humid regions, such as Australia, Guinea, and Brazil, and it can be purified and refined to make the metal. After the first purification step, hydrated aluminum oxide (Al2O3 . xH2O) is obtained. When 100.0 g of this solid were heated, and the water driven off, 65.36 g of Al2O3 remained. How many water molecules (x in the molecular formula) were there in the hydrate?
(Multiple Choice)
4.8/5  (35)
(35)
A gallon of water has a mass of 3.79 kg. How many moles of water (18.02 g/mol) is this?
(Multiple Choice)
4.7/5  (33)
(33)
Household bleach contains 5.0% sodium hypochlorite (NaOCl) by mass. Household ammonia contains 5.0% NH3 by mass. If 50.00 g of bleach are mixed with 50.00 g of 5.0% ammonia, what mass of the toxic chloramine gas would be produced by the following reaction? NaOCl 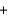 NH3
NH3  NH2Cl
NH2Cl 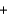 NaOH
NaOH
(Multiple Choice)
4.9/5  (27)
(27)
A hydrocarbon molecule contains carbon and hydrogen atoms in equal numbers. Its molar mass is 130.18 g/mol. What is the molecular formula for the hydrocarbon?
(Multiple Choice)
4.8/5  (36)
(36)
A fuel cell works on the same principle as a battery but is continually fed with fuel. In one fuel cell, methanol (CH3OH) enters on one side of the unit and air enters on the other side. Both circulate past electrodes, and a chemical reaction occurs that produces electricity, plus carbon dioxide and water as byproducts. Which balanced chemical reaction equation best represents the reaction that occurs in this fuel cell?
(Multiple Choice)
4.8/5  (36)
(36)
Why is the term formula unit more appropriate for an ionic compound such as sodium chloride, NaCl, than the term molecular formula?
(Essay)
4.7/5  (26)
(26)
Metallic sodium was originally produced by the Deville process, where sodium carbonate and carbon black are heated to 1100 C to produce sodium (as a liquid at that temperature) and carbon monoxide gas, according to the following balanced equation. Na2CO3(s) 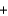 2C(s)
2C(s)  2Na(l)
2Na(l) 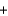 3CO(g)
If 2.3 kg of sodium carbonate is heated, what is the maximum amount of sodium that can be produced?
3CO(g)
If 2.3 kg of sodium carbonate is heated, what is the maximum amount of sodium that can be produced?
(Multiple Choice)
4.8/5  (40)
(40)
The average adult exhales about 1.0 kg of carbon dioxide each day. How much oxygen is needed in metabolizing glucose (C6H12O6, 180 g/mol) to make that much carbon dioxide?
(Multiple Choice)
4.8/5  (32)
(32)
Which statement A-D regarding the carbon cycle is not correct?
(Multiple Choice)
4.8/5  (37)
(37)
What are the stoichiometric coefficients for oxygen and water, respectively, in the balanced chemical reaction equation representing the combustion of butane (C4H10)?
(Multiple Choice)
4.8/5  (32)
(32)
Baking ammonia or ammonium bicarbonate (NH4HCO3, 79.1 g/mol) is a leavening agent used in some older recipes. As it is heated, it breaks down into three gases: ammonia, water, and carbon dioxide. For each 20 g of baking ammonia heated (about 4 tsp), how many grams of carbon dioxide are produced?
(Multiple Choice)
4.9/5  (35)
(35)
Hydrogen peroxide decomposes to produce water and oxygen. Which relationship regarding the quantities of reactants and products associated with this reaction is not correct? 2H2O2 2H2O + O2
(Multiple Choice)
4.9/5  (29)
(29)
Showing 61 - 80 of 141
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)