Exam 6: Making Life Work: Capturing and Using Energy
Exam 1: Life: Chemical, Cellular, and Evolutionary Foundations160 Questions
Exam 2: The Molecules of Life232 Questions
Exam 3: Nucleic Acids and Transcription186 Questions
Exam 4: Translation and Protein Structure148 Questions
Exam 5: Organizing Principles: Lipids, Membranes, and Cell Compartments193 Questions
Exam 6: Making Life Work: Capturing and Using Energy152 Questions
Exam 7: Cellular Respiration: Harvesting Energy From Carbohydrates and Other Fuel Molecules203 Questions
Exam 8: Photosynthesis: Using Sunlight to Build Carbohydrates204 Questions
Exam 9: Cell Signaling148 Questions
Exam 10: Cell and Tissue Architecture: Cytoskeleton, Cell Junctions, and Extracellular Matrix145 Questions
Exam 11: Cell Division: Variations, Regulation, and Cancer169 Questions
Exam 12: Dna Replication and Manipulation169 Questions
Exam 13: Genomes193 Questions
Exam 14: Mutation and Dna Repair165 Questions
Exam 15: Genetic Variation172 Questions
Exam 16: Mendelian Inheritance191 Questions
Exam 17: Inheritance of Sex Chromosomes, Linked Genes, and Organelles201 Questions
Exam 18: The Genetic and Environmental Basis of Complex Traits164 Questions
Exam 19: Genetic and Epigenetic Regulation189 Questions
Exam 20: Genes and Development201 Questions
Exam 21: Evolution: How Genotypes and Phenotypes Change Over Time182 Questions
Exam 22: Species and Speciation132 Questions
Exam 23: Evolutionary Patterns: Phylogeny and Fossils154 Questions
Exam 24: Human Origins and Evolution178 Questions
Exam 25: Cycling Carbon116 Questions
Exam 26: Bacteria and Archaea186 Questions
Exam 27: Eukaryotic Cells: Origins and Diversity153 Questions
Exam 28: Being Multicellular163 Questions
Exam 29: Plant Structure and Function: Moving Photosynthesis Onto Land179 Questions
Exam 30: Plant Reproduction: Finding Mates and Dispersing Young146 Questions
Exam 31: Plant Growth and Development187 Questions
Exam 32: Plant Defense: Keeping the World Green164 Questions
Exam 33: Plant Diversity148 Questions
Exam 34: Fungi: Structure, Function, and Diversity135 Questions
Exam 35: Animal Nervous Systems157 Questions
Exam 36: Animal Sensory Systems and Brain Function205 Questions
Exam 37: Animal Movement: Muscles and Skeletons175 Questions
Exam 38: Animal Endocrine Systems126 Questions
Exam 39: Animal Cardiovascular and Respiratory Systems153 Questions
Exam 40: Animal Metabolism, Nutrition, and Digestion172 Questions
Exam 41: Animal Renal Systems: Water and Waste150 Questions
Exam 42: Animal Reproduction and Development196 Questions
Exam 43: Animal Immune Systems169 Questions
Exam 44: Animal Diversity195 Questions
Exam 45: Animal Behavior186 Questions
Exam 46: Population Ecology132 Questions
Exam 47: Species Interactions, Communities, and Ecosystems178 Questions
Exam 48: Biomes and Global Ecology126 Questions
Exam 49: The Anthropocene: Humans As a Planetary Force192 Questions
Select questions type
Phosphofructokinase is an allosteric enzyme in the pathway that breaks down glucose to produce ATP. Regulation of glycolysis is directly related to the level of ATP and citrate. If ATP or citrate levels are high, phosphofructokinase is inhibited. What will happen to this enzyme when ATP levels drop?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following type(s) of inhibitor can bind to the active site of an enzyme?
(Multiple Choice)
4.9/5  (36)
(36)
Plants power most of their cellular processes by breaking down the sugar they make.
(True/False)
4.9/5  (34)
(34)
A given enzyme in a pathway can be activated by one molecule and inhibited by a different molecule.
(True/False)
4.8/5  (41)
(41)
ATP is a nucleotide composed of adenine, _____, and three phosphate groups.
(Short Answer)
4.8/5  (39)
(39)
The figure below shows a three-dimensional model of the enzyme lysozyme (A) and a map of the location of the important binding and catalytic amino acids that participate at the active site (B). Why are the amino acids of an enzyme that participate in substrate binding and in catalytic activity widely separated on the polypeptide chain, rather than being close together in the primary sequence? 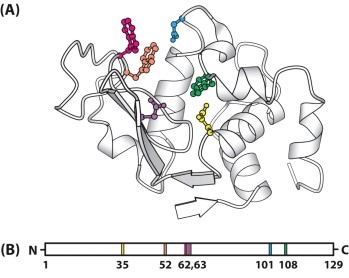
(Essay)
4.7/5  (36)
(36)
Imagine that a researcher tries to reduce the size of an enzyme by removing all the amino acids from the protein except those flankingand constitutingthe active site. Why would this NOT work?
(Multiple Choice)
4.9/5  (39)
(39)
Which of these equations is/are endergonic? Equation 1: ATP + H2O ADP + Pi
G1 = -7 kcal/mol
Equation 2: phosphoenolpyruvate + H2O pyruvate + Pi
G2 = -14.8 kcal/mol
Equation 3: glucose + Pi glucose-6-phosphate + H2O
G3 = +3.3 kcal/mol
Equation 4: ADP + Pi ATP + H2O
G3 = +7 kcal/mol
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements is a consequence of the first law of thermodynamics?
(Multiple Choice)
4.7/5  (43)
(43)
Imagine that a farmer has applied a pesticide to his crops. This pesticide contains an inhibitor that targets an enzyme in beetles. What types of beetles will he find on his farm after three years of pesticide use?
(Multiple Choice)
4.9/5  (34)
(34)
It is often stated that the phosphate bonds in ATP are "high energy," but in fact, they are not notably high in energy. Rather, they are easy to break, and the G of hydrolysis is a "useful" quantity of energy. What makes the phosphate bonds easy to break?
(Multiple Choice)
5.0/5  (43)
(43)
As atmospheric carbon dioxide levels increase, the oceans become more:
(Multiple Choice)
4.8/5  (32)
(32)
A biologist working in a lab adds a compound to a solution that contains an enzyme and substrate. This compound binds to the enzyme and decreases the rate at which the enzyme converts substrate to product. This decrease in enzyme activity persists even if the concentration of substrate in the reaction mix is increased. Therefore, which of the following is TRUE of the compound?
(Multiple Choice)
4.9/5  (33)
(33)
You have entered a 5K race. As you finish the last 50 yards, you are breathing harder than at the start of the race and sweating profusely. This is an example of the _____ law of thermodynamics because _____ is increasing.
(Multiple Choice)
4.8/5  (28)
(28)
A cellular reaction with a G of 8.5 kcal/mol could be effectively coupled to the hydrolysis of a single molecule of ATP.
(True/False)
4.9/5  (38)
(38)
Showing 121 - 140 of 152
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)