Exam 17: Equilibrium the Extent of Chemical Reactions
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
In a chemical reaction, if the starting concentrations of reactants are increased, then the equilibrium constant Kc will also increase.
(True/False)
4.8/5  (35)
(35)
Nitric oxide and bromine were allowed to react in a sealed container. When equilibrium was reached PNO = 0.526 atm, 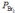 = 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
2NO(g) + Br2(g)
= 1.59 atm, and PNOBr = 7.68 atm. Calculate Kp for the reaction.
2NO(g) + Br2(g)  2NOBr(g)
2NOBr(g)
(Multiple Choice)
4.8/5  (45)
(45)
H2SO3(aq)  HSO3(aq) + H+(aq) Kc = 1.4 × 10-2 H2SO3(aq)
HSO3(aq) + H+(aq) Kc = 1.4 × 10-2 H2SO3(aq)  SO32-(aq) + 2H+(aq) Kc = 9.1 × 10-10
Given the above equilibrium constant data at 25°C, what is the value of Kc at this temperature for the reaction
HSO3-(aq)
SO32-(aq) + 2H+(aq) Kc = 9.1 × 10-10
Given the above equilibrium constant data at 25°C, what is the value of Kc at this temperature for the reaction
HSO3-(aq)  SO32-(aq) + H+(aq)?
SO32-(aq) + H+(aq)?
(Multiple Choice)
4.8/5  (39)
(39)
Consider the following two equilibria and their respective equilibrium constants: (1) NO(g) + ½O2(g)  NO2(g)
(2) 2NO2(g)
NO2(g)
(2) 2NO2(g)  2NO(g) + O2(g)
Which one of the following is the correct relationship between the equilibrium constants K1 and K2?
2NO(g) + O2(g)
Which one of the following is the correct relationship between the equilibrium constants K1 and K2?
(Multiple Choice)
4.7/5  (41)
(41)
A mixture of 0.500 mole of carbon monoxide and 0.400 mole of bromine was placed into a rigid 1.00-L container and the system was allowed to come to equilibrium. The equilibrium
Concentration of COBr2 was 0.233 M. What is the value of Kc for this reaction?
CO(g) + Br2(g)  COBr2(g)
COBr2(g)
(Multiple Choice)
5.0/5  (42)
(42)
A chemical reaction has an equilibrium constant of 2 × 106. If this reaction is at equilibrium, select the one correct conclusion that can be made about the reaction.
(Multiple Choice)
4.7/5  (52)
(52)
Which of the following has an effect on the magnitude of the equilibrium constant?
(Multiple Choice)
4.8/5  (28)
(28)
Write the mass-action expression,  c, for the following chemical reaction equation.
2C6H6(g) + 15O2(g)
c, for the following chemical reaction equation.
2C6H6(g) + 15O2(g)  12CO2(g) + 6H2O(g)
12CO2(g) + 6H2O(g)
(Multiple Choice)
4.9/5  (38)
(38)
If all of the coefficients in the balanced equation for an equilibrium reaction are doubled, then the value of the equilibrium constant, Kc, will also be doubled.
(True/False)
4.7/5  (31)
(31)
Sodium hydrogen carbonate decomposes above 110°C to form sodium carbonate, water, and carbon dioxide. 2NaHCO3(s)  Na2CO3(s) + H2O(g) + CO2(g)
One thousand grams of sodium hydrogen carbonate are added to a reaction vessel, the temperature is increased to 200°C, and the system comes to equilibrium. What happens in this system if another 50 g of sodium carbonate are now added?
Na2CO3(s) + H2O(g) + CO2(g)
One thousand grams of sodium hydrogen carbonate are added to a reaction vessel, the temperature is increased to 200°C, and the system comes to equilibrium. What happens in this system if another 50 g of sodium carbonate are now added?
(Multiple Choice)
4.8/5  (33)
(33)
What is the mass-action expression,  c, for the following chemical reaction? Cu2+(aq) + 4NH3(aq)
c, for the following chemical reaction? Cu2+(aq) + 4NH3(aq)  Cu(NH3)42+(aq)
Cu(NH3)42+(aq)
(Multiple Choice)
4.7/5  (39)
(39)
Chemical reactions generally reach equilibrium because one of the reactants is used up.
(True/False)
4.9/5  (35)
(35)
The following reaction is at equilibrium in a closed container. CuSO4.5H2O(s)  CuSO4(s) + 5H2O(g)
Which, if any, of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
CuSO4(s) + 5H2O(g)
Which, if any, of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
(Multiple Choice)
4.9/5  (38)
(38)
Ammonium iodide dissociates reversibly to ammonia and hydrogen iodide. NH4I(s)  NH3(g) + HI(g)
At 400°C, Kp = 0.215. Calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400°C.
NH3(g) + HI(g)
At 400°C, Kp = 0.215. Calculate the partial pressure of ammonia at equilibrium when a sufficient quantity of ammonium iodide is heated to 400°C.
(Multiple Choice)
4.8/5  (36)
(36)
Methanol can be synthesized by combining carbon monoxide and hydrogen. CO(g) + 2H2(g)  CH3OH(g)
A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm. What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
CH3OH(g)
A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm. What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
(Multiple Choice)
4.9/5  (33)
(33)
The equilibrium constant for reaction (1) below is 276. Under the same conditions, what is the equilibrium constant of reaction (2)? (1) ½X2(g) + ½Y2(g)  XY(g)
(2) 2XY(g)
XY(g)
(2) 2XY(g)  X2(g) + Y2(g)
X2(g) + Y2(g)
(Multiple Choice)
5.0/5  (37)
(37)
10.0 mL of a 0.100 mol L-1 solution of a metal ion M2+ is mixed with 10.0 mL of a 0.100 mol L-1 solution of a substance L. The following equilibrium is established: M2+(aq) + 2L(aq)  ML22+(aq)
At equilibrium the concentration of L is found to be 0.0100 mol L-1. What is the equilibrium concentration of ML22+, in mol L-1?
ML22+(aq)
At equilibrium the concentration of L is found to be 0.0100 mol L-1. What is the equilibrium concentration of ML22+, in mol L-1?
(Multiple Choice)
4.8/5  (48)
(48)
Magnesium hydroxide is used in several antacid formulations. When it is added to water it dissociates into magnesium and hydroxide ions. Mg(OH)2(s)  Mg2+(aq) + 2OH-(aq)
The equilibrium constant at 25°C is 8.9 × 10-12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH)2 are now added to the mixture?
Mg2+(aq) + 2OH-(aq)
The equilibrium constant at 25°C is 8.9 × 10-12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH)2 are now added to the mixture?
(Multiple Choice)
4.8/5  (38)
(38)
Increasing the initial amount of the limiting reactant in a reaction will increase the value of the equilibrium constant, Kc.
(True/False)
4.9/5  (38)
(38)
The equilibrium constant for the reaction of bromine with chlorine to form bromine monochloride is 58.0 at a certain temperature. Br2(g) + Cl2(g)  2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g)
2BrCl(g)
What is the equilibrium constant for the following reaction?
BrCl(g)  ½Br2(g) + ½Cl2(g)
½Br2(g) + ½Cl2(g)
(Multiple Choice)
4.8/5  (41)
(41)
Showing 41 - 60 of 97
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)