Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Rank H3PO4,H2PO4-,and HPO42- in order of increasing acid strength.
(Multiple Choice)
4.8/5  (38)
(38)
The autoionization of pure water,as represented by the equation below,is known to be endothermic (ΔrΗ > 0).Which of the following correctly states what occurs as the temperature of pure water is raised? H2O(l)+ H2O(l)  H3O+(aq)+ OH-(aq)ΔrΗ > 0
H3O+(aq)+ OH-(aq)ΔrΗ > 0
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following is the correct hydronium ion ,H3O+,concentration of 0.0013 M KOH(aq)at 25 °C? (Kw = 1.01 × 10-14)
(Multiple Choice)
4.9/5  (39)
(39)
What is the equilibrium concentration of H2C2O4 in a 0.170 M oxalic acid,H2C2O4,solution? For oxalic acid,Ka1 = 5.6 × 10-2 and Ka2 = 5.1 × 10-5.
(Multiple Choice)
4.8/5  (32)
(32)
A solution has a hydroxide ion concentration of 0.0040 M.Calculate the pOH of the solution at 25 °C.(Kw = 1.01 × 10-14)
(Multiple Choice)
4.8/5  (32)
(32)
What is the hydronium-ion concentration in a solution formed by combining 750 mL of 0.10 M NaOH with 250 mL of 0.30 M HCl? NaOH(aq)+ HCl(aq)→ NaCl(aq)+ H2O(l)
(Multiple Choice)
5.0/5  (41)
(41)
What is the hydroxide-ion concentration of a 0.190 M sodium oxalate (Na2C2O4)solution? For oxalic acid (H2C2O4),Ka1 = 5.6 × 10-2 and Ka2 = 5.1 × 10-5.(Kw = 1.01 × 10-14)
(Multiple Choice)
5.0/5  (44)
(44)
The chemical equations below show the reaction of Al(OH)3 as a Lewis acid and as a Lewis base,respectively.
Al(OH)3(s)+ OH-(aq) 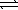 Al(OH)4-(aq)
Al(OH)3(s)+ 3 H3O+(aq)
Al(OH)4-(aq)
Al(OH)3(s)+ 3 H3O+(aq) 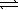 Al3+(aq)+ 3 H2O(
Al3+(aq)+ 3 H2O( 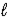 )
Substances that can behave as either Lewis acids or bases are called ________ substances.
)
Substances that can behave as either Lewis acids or bases are called ________ substances.
(Short Answer)
4.8/5  (30)
(30)
What is the hydronium-ion concentration of a 0.25 M solution of HCN (Ka = 4.9 × 10-10)at 25°C?
(Multiple Choice)
4.8/5  (36)
(36)
Which are the Brønsted-Lowry bases in the following equilibrium? CH3COO-(aq)+ H2O(l)  CH3COOH(aq)+ OH-(aq)
CH3COOH(aq)+ OH-(aq)
(Multiple Choice)
4.8/5  (40)
(40)
Consider the Ka values for the following acids: Cyanic acid,HOCN,3.5 × 10-4
Formic acid,HCHO2,1.7 × 10-4
Lactic acid,HC3H5O3,1.3 × 10-4
Propionic acid,HC3H5O2,1.3 × 10-5
Benzoic acid,HC7H5O2,6.3 × 10-5
Which of the following is the strongest acid?
(Multiple Choice)
4.9/5  (39)
(39)
When a Lewis acid combines with a Lewis base,the base supplies both the electrons to the bond.This type of chemical bond is called a(n)_____ covalent bond.
(Short Answer)
4.8/5  (42)
(42)
Consider the Ka values for the following acids: Cyanic acid,HOCN,3.5 × 10-4
Formic acid,HCHO2,1.7 × 10-4
Lactic acid,HC3H5O3,1.3 × 10-4
Propionic acid,HC3H5O2,1.3 × 10-5
Benzoic acid,HC7H5O2,6.3 × 10-5
Given initially equimolar solutions of each weak acid,which solution will have the highest pH once equilibrium is established?
(Multiple Choice)
4.7/5  (36)
(36)
What is the pH of 0.010 M aqueous hypochlorous acid? (Ka of HOCl = 3.5 × 10-8)
(Multiple Choice)
4.8/5  (34)
(34)
The pH of aqueous 0.10 M pyridine (C5H5N)ion is 9.09.What is the Kb of this base?
(Multiple Choice)
4.8/5  (29)
(29)
H3PO3 is a diprotic weak acid.What is the balanced equilibrium defined as Kb2 of H3PO3?
(Multiple Choice)
4.9/5  (34)
(34)
Brønsted-Lowry acid-base reactions are restricted to only aqueous solutions.
(Multiple Choice)
4.8/5  (30)
(30)
The pH of a solution at 25 °C in which [OH-] = 3.9 × 10-5 M is _____.(Kw = 1.01 × 10-14)
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following substances is never a Brønsted-Lowry base in an aqueous solution?
(Multiple Choice)
4.8/5  (43)
(43)
Showing 41 - 60 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)