Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Which of the following ionic compounds does not produce a basic aqueous solution at 25 °C?
(Multiple Choice)
5.0/5  (41)
(41)
Aqueous solutions of ammonia (NH3)and hydrogen cyanide (HCN)react to produce ammonium cyanide,(NH4CN)according to the following equilibrium reaction. NH3(aq)+ HCN(aq)D NH4+(aq)+ CN−(aq)
Given the following equilibrium constants,which statement best describes the reaction once equilibrium is established? (Kw = 1.01 × 10-14)
NH4+
Ka = 5.6 × 10-10
HCN
Ka = 4.0 × 10-10
(Multiple Choice)
4.8/5  (39)
(39)
At 50°C the autoionization constant for pure water,Kw,is 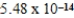 .What is the H3O+ concentration in pure water at 50°C?
.What is the H3O+ concentration in pure water at 50°C?
(Multiple Choice)
4.8/5  (37)
(37)
What is the H3O+ concentration in 0.0072 M NaOH(aq)at 25 °C? (Kw = 1.01 × 10-14)
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following expressions is not equivalent to the formula of pH?
(Multiple Choice)
4.9/5  (26)
(26)
Carbonic acid is a diprotic acid,H2CO3,with Ka1 = 4.2 × 10-7 and Ka2 = 4.8 × 10-11 at 25°C.The ion product for water is Kw = 1.0 × 10-14 at 25°C.What is the OH- concentration of a solution that is 0.39 M in Na2CO3?
(Multiple Choice)
4.8/5  (31)
(31)
The equilibrium constant,Ka,for a monoprotic acid (benzoic acid)is 6.3 × 10-5.Which of the following is the correct value of Kb for the benzoate ion,the conjugate base of benzoic acid?
(Multiple Choice)
4.9/5  (41)
(41)
Molecules or ions that can alternately behave as either a Brønsted-Lowry acid or base are called
(Multiple Choice)
4.9/5  (36)
(36)
What is the equilibrium hydronium ion concentration of an initially 4.5 M solution of hypoiodous acid,HOI,at 25°C (Ka =  )?
)?
(Multiple Choice)
4.7/5  (36)
(36)
Calculate the pH of a 1.7 M solution of H2A (Ka1 = 1.0 × 10-6 and Ka2 is 1.0 × 10-10).
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following is the correct hydroxide ion concentration in 0.48 M CH3CO2-(aq)? (Kb of CH3CO2- = 5.6 × 10-10)
(Multiple Choice)
4.8/5  (40)
(40)
At 25 °C,all of the following ions produce an acidic solution,except ____.
(Multiple Choice)
4.9/5  (44)
(44)
Which one of the following aqueous solutions will have a pH of 2.00 at 25 °C? (Kw = 1.01 × 10-14)
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following is true of the equilibrium constants (K,Ka,Kb)?
(Multiple Choice)
4.9/5  (27)
(27)
The complete reaction of an acid and base is as follows. HNO3(aq)+ LiOH(aq)→ H2O( 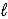 )+ LiNO3(aq)
What is the equilibrium constant for the net ionic reaction at 25 °C?
)+ LiNO3(aq)
What is the equilibrium constant for the net ionic reaction at 25 °C?
(Multiple Choice)
4.8/5  (37)
(37)
Given the following equilibrium constants, Ka (HSO4-)= 1.2 × 10-2
Kb (CH3CO2-)= 5.6 × 10-10
Kw = 1.00 × 10-14
determine the equilibrium constant for the reaction below at 25 °C.
HSO4-(aq)+ CH3CO2-(aq) 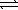 SO42-(aq)+ CH3CO2H(aq)
SO42-(aq)+ CH3CO2H(aq)
(Multiple Choice)
4.8/5  (35)
(35)
All of the following compounds are acids containing chlorine.Which compound is the weakest acid?
(Multiple Choice)
4.9/5  (33)
(33)
What is the pH of a 0.33 M solution of methylamine (CH3NH2,Kb = 4.4 × 10-4)at 25oC? (Kw = 1.01 × 10-14)
(Multiple Choice)
4.8/5  (39)
(39)
When a Lewis acid and a Lewis base combine,the product may be referred to as an acid-base ________.
(Short Answer)
4.9/5  (34)
(34)
Showing 61 - 80 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)