Exam 16: Principles of Chemical Reactivity: Equilibria
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
A sample of solid NH4NO3 was placed in an evacuated container and then heated so that it decomposed explosively according to the following equation: NH4NO3(s)  N2O(g)+ 2H2O(g)
At equilibrium the total pressure in the container was found to be 2.72 atm at a temperature of 500.°C.Calculate Kp.
N2O(g)+ 2H2O(g)
At equilibrium the total pressure in the container was found to be 2.72 atm at a temperature of 500.°C.Calculate Kp.
(Multiple Choice)
4.8/5  (36)
(36)
For the equilibrium N2O4(g) 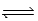 2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.7 × 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium?
2NO2(g),at 298 K,Kp = 0.15.For this reaction system,it is found that the partial pressure of N2O4 is 3.7 × 10-2 atm at equilibrium.What is the partial pressure of NO2 at equilibrium?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following is the correct balanced equation for the equilibrium constant expression given below? 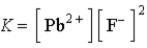
(Multiple Choice)
4.9/5  (38)
(38)
At 25 °C,0.138 mg AgBr dissolves in 10.0 L of water.What is the equilibrium constant for the reaction below? AgBr(s) 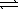 Ag+(aq)+ Br-(aq)
Ag+(aq)+ Br-(aq)
(Multiple Choice)
4.9/5  (33)
(33)
Consider the following equilibrium: CO2(g)+ H2(g)  CO(g)+ H2O(g); Kc = 1.6 at 1260 K
Suppose 0.019 mol CO2 and 0.030 mol H2 are placed in a 3.00-L vessel at 1260 K.What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L · atm/K·mol)
CO(g)+ H2O(g); Kc = 1.6 at 1260 K
Suppose 0.019 mol CO2 and 0.030 mol H2 are placed in a 3.00-L vessel at 1260 K.What is the equilibrium partial pressure of CO(g)? (R = 0.0821 L · atm/K·mol)
(Multiple Choice)
4.9/5  (39)
(39)
The following reaction occurred when a 1.0-liter reaction vessel was initially charged with 2.0 moles of N2(g)and 4.0 moles of H2(g): 3H2(g)+ N2(g)  2NH3(g)
Once equilibrium was established,the concentration of NH3(g)was determined to be 0.57 M at 700.°C.The value for Kc at 700.°C for the formation of ammonia is:
2NH3(g)
Once equilibrium was established,the concentration of NH3(g)was determined to be 0.57 M at 700.°C.The value for Kc at 700.°C for the formation of ammonia is:
(Multiple Choice)
4.7/5  (42)
(42)
Consider the formation of ozone by the following reaction. 3 O2(g) 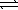 2 O3(g)
Calculate the value of Kp,given that Kc = 2.5 × 10-29 at 298 K.(R = 0.08206 L⋅atm/mol ⋅ K)
2 O3(g)
Calculate the value of Kp,given that Kc = 2.5 × 10-29 at 298 K.(R = 0.08206 L⋅atm/mol ⋅ K)
(Multiple Choice)
4.9/5  (32)
(32)
The standard enthalpy of formation of ammonia is -46.1 kJ/mol.
1/2 N2(g)+ 3/2 H2(g) 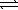 NH3(g)
Commercially,the reaction is carried out at high temperatures.Using your knowledge of kinetics and equilibrium,explain an advantage and a disadvantage of synthesizing ammonia at high temperatures.
NH3(g)
Commercially,the reaction is carried out at high temperatures.Using your knowledge of kinetics and equilibrium,explain an advantage and a disadvantage of synthesizing ammonia at high temperatures.
(Essay)
4.8/5  (46)
(46)
Consider the reaction below. 2 HF(g) 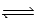 H2(g)+ F2(g)(Kc = 1.00 × 10-2)
Given that 1.00 mol of HF(g),0.241 mol of H2(g),and 0.750 mol of F2(g)are mixed in a 5.00 L flask,determine the reaction quotient,Q.
H2(g)+ F2(g)(Kc = 1.00 × 10-2)
Given that 1.00 mol of HF(g),0.241 mol of H2(g),and 0.750 mol of F2(g)are mixed in a 5.00 L flask,determine the reaction quotient,Q.
(Multiple Choice)
4.7/5  (42)
(42)
Write the expression for Kp for the reaction below. 2 NOBr(g) 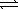 2 NO(g)+ Br2(
2 NO(g)+ Br2( 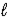 )
)
(Multiple Choice)
4.9/5  (34)
(34)
When gaseous carbon monoxide and hydrogen are combined in a sealed vessel and heated they will eventually form an equilibrium mixture of reactants and products according to the balanced chemical equilibrium below. CO(g)+ 3H2(g) 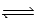 CH4(g)+ H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve B?
CH4(g)+ H2O(g)
In one such reaction 3 moles of one reactant were combined with 1 mole of the other reactant in a fixed volume vessel and heated to 1200 K.Analysis of the reaction mixture at various times gave the results below.Which component of the reaction mixture is represented by curve B? 
(Multiple Choice)
4.8/5  (26)
(26)
Which of the following statements is true if reaction quotient (Q)is greater than equilibrium constant (K)?
(Multiple Choice)
4.7/5  (43)
(43)
For the reaction 2NO(g)+ O2(g) 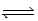 2NO2(g)at 750°C,what is the relationship between Kc and Kp?
2NO2(g)at 750°C,what is the relationship between Kc and Kp?
(Multiple Choice)
4.9/5  (31)
(31)
Consider the following equilibrium. PCl3(g)+ Cl2(g)  PCl5(g) ΔH = -92 kJ
The concentration of PCl3 at equilibrium can be increased by:
PCl5(g) ΔH = -92 kJ
The concentration of PCl3 at equilibrium can be increased by:
(Multiple Choice)
4.8/5  (45)
(45)
Assume that the following chemical reaction is at equilibrium. 2 ICl(g) 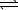 I2(g)+ Cl2(g)
ΔH° = +26.9 kJ
At 25 °C,Kp = 2.0 × 105.If the temperature is increase to 45 °C,which statement applies?
I2(g)+ Cl2(g)
ΔH° = +26.9 kJ
At 25 °C,Kp = 2.0 × 105.If the temperature is increase to 45 °C,which statement applies?
(Multiple Choice)
5.0/5  (41)
(41)
What is the Kc expression for the equilibrium given below? CuI(s) 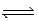 Cu+(aq)+ I−(aq)
Cu+(aq)+ I−(aq)
(Multiple Choice)
4.9/5  (41)
(41)
Excess Ag2SO4(s)is placed in water at 25 °C.At equilibrium,the solution contains 0.029 M Ag+(aq).What is the equilibrium constant for the reaction below? Ag2SO4(s) 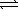 2 Ag+(aq)+ SO42-(aq)
2 Ag+(aq)+ SO42-(aq)
(Multiple Choice)
4.9/5  (35)
(35)
Showing 61 - 77 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)