Exam 16: Principles of Chemical Reactivity: Equilibria
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Which of the following equilibria would not be affected by pressure change at constant temperature?
(Multiple Choice)
4.8/5  (40)
(40)
Nitrosyl bromide decomposes according to the chemical equation below. 2 NOBr(g) 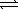 2 NO(g)+ Br2(g)
When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium,22% of the NOBr decomposes.What is the equilibrium constant,Kp,for the reaction?
2 NO(g)+ Br2(g)
When 0.260 atm of NOBr is sealed in a flask and allowed to reach equilibrium,22% of the NOBr decomposes.What is the equilibrium constant,Kp,for the reaction?
(Multiple Choice)
4.9/5  (38)
(38)
At 800 K,the equilibrium constant,Kp,for the following reaction is 3.2 × 10-7. 2 H2S(g) 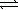 2 H2(g)+ S2(g)
A reaction vessel at 800 K initially contains 3.00 atm of H2S.If the reaction is allowed to equilibrate,what is the equilibrium pressure of S2?
2 H2(g)+ S2(g)
A reaction vessel at 800 K initially contains 3.00 atm of H2S.If the reaction is allowed to equilibrate,what is the equilibrium pressure of S2?
(Multiple Choice)
4.7/5  (38)
(38)
At 700 K,Kp for the following equilibrium is 5.6 × 10-3. 2HgO(s) 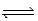 2Hg(l)+ O2(g)
Suppose 43.1 g of mercury(II)oxide is placed in a sealed 4.00-L vessel at 700 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L · atm/(K · mol))
2Hg(l)+ O2(g)
Suppose 43.1 g of mercury(II)oxide is placed in a sealed 4.00-L vessel at 700 K.What is the partial pressure of oxygen gas at equilibrium? (R = 0.0821 L · atm/(K · mol))
(Multiple Choice)
4.8/5  (34)
(34)
For the reaction N2O4(g)  2NO2(g),Kp = 0.148 at a temperature of 298 K.What is Kp for the following reaction? 12NO2(g)
2NO2(g),Kp = 0.148 at a temperature of 298 K.What is Kp for the following reaction? 12NO2(g)  6N2O4(g)
6N2O4(g)
(Multiple Choice)
4.9/5  (35)
(35)
Sulfuryl chloride decomposes to sulfur dioxide and chlorine. SO2Cl2(g) 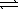 SO2(g)+ Cl2(g)
Kc is 0.045 at 648 K.If an initial concentration of 0.075 M SO2Cl2 is allowed to equilibrate,what is the equilibrium concentration of Cl2?
SO2(g)+ Cl2(g)
Kc is 0.045 at 648 K.If an initial concentration of 0.075 M SO2Cl2 is allowed to equilibrate,what is the equilibrium concentration of Cl2?
(Multiple Choice)
4.9/5  (36)
(36)
Which of the following expressions correctly describes the equilibrium constant Kc for the reaction given below? 2 C2H2(g)+ 5 O2(g) 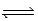 4 CO2(g)+ 2 H2O(g)
4 CO2(g)+ 2 H2O(g)
(Multiple Choice)
4.8/5  (39)
(39)
The equilibrium constant at 25 °C for the dissolution of silver iodide is 8.5 × 10-17. AgI(s) 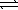 Ag+(aq)+ I-(aq)
If an excess quantity of AgI(s)is added to water and allowed to equilibrate,what is the equilibrium concentration of I-?
Ag+(aq)+ I-(aq)
If an excess quantity of AgI(s)is added to water and allowed to equilibrate,what is the equilibrium concentration of I-?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements about the reaction quotient,Q,is false?
(Multiple Choice)
4.8/5  (50)
(50)
In an experiment,0.46 mol H2 and 0.46 mol I2 are mixed in a 1.00-L container,and the reaction forms HI.If Kc = 49.for this reaction,what is the equilibrium concentration of HI? I2(g)+ H2(g) 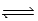 2HI(g)
2HI(g)
(Multiple Choice)
4.7/5  (27)
(27)
At 25 °C,the decomposition of dinitrogen tetraoxide N2O4(g) 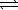 2 NO2(g)
Has an equilibrium constant (Kp)of 0.144.At equilibrium,the total pressure of the system is 0.0758 atm.What is the partial pressure of each gas?
2 NO2(g)
Has an equilibrium constant (Kp)of 0.144.At equilibrium,the total pressure of the system is 0.0758 atm.What is the partial pressure of each gas?
(Multiple Choice)
4.8/5  (37)
(37)
An aqueous mixture of phenol and ammonia has initial concentrations of 0.200 M C6H5OH(aq)and 0.120 M NH3(aq).At equilibrium,the C6H5O-(aq)concentration is 0.050 M.Calculate the equilibrium constant,K,for the reaction below. C6H5OH(aq)+ NH3(aq) 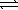 C6H5O- + NH4+(aq)
C6H5O- + NH4+(aq)
(Multiple Choice)
4.9/5  (39)
(39)
Given the following chemical equilibria, N2(g)+ O2(g) 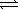 2 NO(g)
K1
N2(g)+ 3 H2(g)
2 NO(g)
K1
N2(g)+ 3 H2(g) 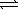 2 NH3(g)
K2
H2(g)+ 1/2 O2(g)
2 NH3(g)
K2
H2(g)+ 1/2 O2(g) 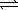 H2O(g)
K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g)+ 5 O2(g)
H2O(g)
K3
Determine the method used to calculate the equilibrium constant for the reaction below.
4 NH3(g)+ 5 O2(g) 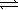 4 NO(g)+ 6 H2O(g)
K c
4 NO(g)+ 6 H2O(g)
K c
(Multiple Choice)
4.9/5  (32)
(32)
For the equilibrium PCl5(g) 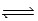 PCl3(g)+ Cl2(g),Kc = 2.0 × 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.50 M,what is the equilibrium concentration of PCl5(g)?
PCl3(g)+ Cl2(g),Kc = 2.0 × 101 at 240°C.If pure PCl5 is placed in a 1.00-L container and allowed to come to equilibrium,and the equilibrium concentration of PCl3(g)is 0.50 M,what is the equilibrium concentration of PCl5(g)?
(Multiple Choice)
4.7/5  (40)
(40)
Given the following chemical equilibrium COCl2(g) 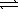 CO(g)+ Cl2(g),calculate the value of Kc when Kp = 6.5 × 1011 at 298 K.(R = 0.08206 L⋅atm/mol ⋅ K)
CO(g)+ Cl2(g),calculate the value of Kc when Kp = 6.5 × 1011 at 298 K.(R = 0.08206 L⋅atm/mol ⋅ K)
(Multiple Choice)
5.0/5  (40)
(40)
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and allowed to reach equilibrium described by the equation N2O4(g) 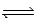 2NO2(g). If at equilibrium the N2O4 is 27.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
2NO2(g). If at equilibrium the N2O4 is 27.0% dissociated,what is the value of the equilibrium constant,Kc,for the reaction under these conditions?
(Multiple Choice)
4.8/5  (36)
(36)
If the reaction quotient,Q,is greater than K in a gas phase reaction,then
(Multiple Choice)
4.9/5  (42)
(42)
If a stress is applied to an equilibrium system,the system will respond in such a way as to relieve that stress.This is a statement of ________ principle.
(Short Answer)
4.8/5  (39)
(39)
Consider the reaction H2 + I2  2HI for which Kc = 44.0 at a high temperature.If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium,determine the equilibrium concentration of the hydrogen.
2HI for which Kc = 44.0 at a high temperature.If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium,determine the equilibrium concentration of the hydrogen.
(Multiple Choice)
4.9/5  (38)
(38)
Showing 41 - 60 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)