Exam 16: Principles of Chemical Reactivity: Equilibria
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Which of the following expressions for K is correct for the reaction given below? HF(aq)+ H2O( 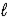 )
) 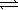 F-(aq)+ H3O+(aq)
F-(aq)+ H3O+(aq)
(Multiple Choice)
4.8/5  (39)
(39)
When 0.20 mole HF is dissolved in water to a volume of 1.00 L,5.8% of the HF dissociates to form F-(aq).What is the equilibrium constant for the reaction? HF(aq)+ H2O( 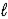 )
) 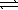 F-(aq)+ H3O+(aq)
F-(aq)+ H3O+(aq)
(Multiple Choice)
4.8/5  (43)
(43)
A 3.50-mol sample of HI is placed in a 1.00-L vessel at 460°C,and the reaction system is allowed to come to equilibrium.The HI partially decomposes,forming 0.266 mol H2 and 0.266 mol I2 at equilibrium.What is the equilibrium constant Kc for the following reaction at 460°C? ½ H2(g)+ ½ I2(g) 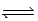 HI(g)
HI(g)
(Multiple Choice)
4.8/5  (39)
(39)
The equilibrium constant,K,is always the same within experimental error for all experiments done at a given temperature.
(True/False)
5.0/5  (34)
(34)
A 10.0 g sample of solid NH4Cl is heated in a 5.00 L container to 900 °C.At equilibrium,the pressure of NH3(g)is 1.17 atm.Calculate the equilibrium constant,Kp,for the reaction below. NH4Cl(s)  NH3(g)+ HCl(g)
NH3(g)+ HCl(g)
(Multiple Choice)
4.7/5  (43)
(43)
The thermochemical equation for the formation of ammonia from elemental nitrogen and hydrogen is as follows. N2(g)+ 3 H2(g) 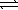 2 NH3(g)
ΔH = -92.2 kJ
Given a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
2 NH3(g)
ΔH = -92.2 kJ
Given a system that is initially at equilibrium,which of the following actions cause the reaction to proceed to the left?
(Multiple Choice)
4.8/5  (39)
(39)
Consider the following equilibrium: C2H6(g)+ C5H12(g) 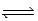 CH4(g)+ C6H14(g); Kp = 9.57 at 500 K
Suppose 22.7 g each of CH4,C2H6,C5H12,and C6H14 are placed in a 35.0-L reaction vessel at 500 K.Which of the following statements is correct?
CH4(g)+ C6H14(g); Kp = 9.57 at 500 K
Suppose 22.7 g each of CH4,C2H6,C5H12,and C6H14 are placed in a 35.0-L reaction vessel at 500 K.Which of the following statements is correct?
(Multiple Choice)
4.9/5  (35)
(35)
A mixture of nitrogen and hydrogen was allowed to come to equilibrium at a given temperature. 3H2 + N2  2NH3
An analysis of the mixture at equilibrium revealed 2.1 mol N2,2.8 mol H2,and 1.8 mol NH3.How many moles of H2 were present at the beginning of the reaction?
2NH3
An analysis of the mixture at equilibrium revealed 2.1 mol N2,2.8 mol H2,and 1.8 mol NH3.How many moles of H2 were present at the beginning of the reaction?
(Multiple Choice)
4.9/5  (43)
(43)
Nitrogen trifluoride decomposes at to form nitrogen and fluorine gases according to the following equation: 2NF3(g) 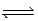 N2(g)+ 3F2(g)
2)50-L reaction vessel is initially charged with 1.22 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0194 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L⋅atm/mol⋅K)
N2(g)+ 3F2(g)
2)50-L reaction vessel is initially charged with 1.22 mol of NF3 and allowed to come to equilibrium at 800 K.Once equilibrium is established,the reaction vessel is found to contain 0.0194 mol of N2.What is the value of Kp at this temperature? (R = 0.0821 L⋅atm/mol⋅K)
(Multiple Choice)
4.8/5  (46)
(46)
When the pressure of an equilibrium mixture of SO2,O2,and SO3 is doubled at constant temperature,what the effect on Kp? 2SO2(g)+ O2(g)  2SO3(g)
2SO3(g)
(Multiple Choice)
4.9/5  (32)
(32)
If Kc = 0.124 for A2 + 2B 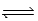 2AB,what is the value of Kc for the reaction 4AB
2AB,what is the value of Kc for the reaction 4AB 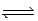 2A2 + 4B?
2A2 + 4B?
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following is the correct balanced equation for the equilibrium constant expression given below? 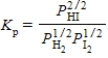
(Multiple Choice)
4.7/5  (34)
(34)
A 3.00-liter flask initially contains 3.00 mol of gas A and 1.50 mol of gas B.Gas A decomposes according to the following reaction: 3A  2B + C
The equilibrium concentration of gas C is 0.134 mol/L.Determine the value of the equilibrium constant,Kc.
2B + C
The equilibrium concentration of gas C is 0.134 mol/L.Determine the value of the equilibrium constant,Kc.
(Multiple Choice)
4.9/5  (35)
(35)
At a high temperature,equal concentrations of 0.160 mol/L of H2(g)and I2(g)are initially present in a flask.The H2 and I2 react according to the balanced equation below. H2(g)+ I2(g) 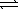 2 HI(g)
When equilibrium is reached,the concentration of H2(g)has decreased to 0.036 mol/L.What is the equilibrium constant,Kc,for the reaction?
2 HI(g)
When equilibrium is reached,the concentration of H2(g)has decreased to 0.036 mol/L.What is the equilibrium constant,Kc,for the reaction?
(Multiple Choice)
4.9/5  (38)
(38)
Given the equilibrium constants for the equilibria, NH4+(aq)+ H2O(l) 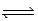 NH3(aq)+ H3O+(aq); Kc =
NH3(aq)+ H3O+(aq); Kc = 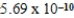 2H2O(l)
2H2O(l) 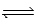 2H3O+(aq); Kc =
2H3O+(aq); Kc = 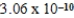 determine Kc for the following equilibrium.
CH3COOH(aq)+ NH3(aq)
determine Kc for the following equilibrium.
CH3COOH(aq)+ NH3(aq) 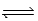 CH3COO−(aq)+ NH4+(aq)
CH3COO−(aq)+ NH4+(aq)
(Multiple Choice)
4.7/5  (41)
(41)
Nitrogen and oxygen gases may react to form nitrogen monoxide.At 1500 °C,Kc equals 1.0 × 10−5. N2(g)+ O2(g) 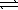 2 NO(g)
If 0.030 mol N2 and 0.030 mol O2 are sealed in a 1.0 L flask at 1500 °C,what is the concentration of NO(g)when equilibrium is established?
2 NO(g)
If 0.030 mol N2 and 0.030 mol O2 are sealed in a 1.0 L flask at 1500 °C,what is the concentration of NO(g)when equilibrium is established?
(Multiple Choice)
4.8/5  (40)
(40)
Given the following equilibria, Ni2+(aq)+ 2 OH-(aq) 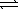 Ni(OH)2(s)
K1 = 1.8 × 1015
Ni2+(aq)+ 4 CN-(aq)
Ni(OH)2(s)
K1 = 1.8 × 1015
Ni2+(aq)+ 4 CN-(aq) 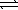 Ni(CN)42-(aq)
K2 = 2.0 × 1031
Determine the equilibrium constant,Kc,for the following reaction.
Ni(OH)2(s)+ 4 CN-(aq)
Ni(CN)42-(aq)
K2 = 2.0 × 1031
Determine the equilibrium constant,Kc,for the following reaction.
Ni(OH)2(s)+ 4 CN-(aq) 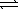 Ni(CN)42-(aq)+ 2 OH-(aq)
Ni(CN)42-(aq)+ 2 OH-(aq)
(Multiple Choice)
5.0/5  (33)
(33)
Consider the following equilibria. PbBr2(s) 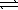 Pb2+(aq)+ 2 Br-(aq)
K1 = 6.6 × 10-6
Pb(OH)2(s)
Pb2+(aq)+ 2 Br-(aq)
K1 = 6.6 × 10-6
Pb(OH)2(s) 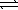 Pb2+(aq)+ 2 OH-(aq)
K2 = 1.4 × 10-15
Determine the equilibrium constant,Kc,for the reaction below.
PbBr2(s)+ 2 OH-(aq)
Pb2+(aq)+ 2 OH-(aq)
K2 = 1.4 × 10-15
Determine the equilibrium constant,Kc,for the reaction below.
PbBr2(s)+ 2 OH-(aq) 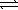 Pb(OH)2(s)+ 2 Br-(aq)
Pb(OH)2(s)+ 2 Br-(aq)
(Multiple Choice)
4.7/5  (34)
(34)
Showing 21 - 40 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)