Exam 13: The Solid State
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Which of the compounds below is not an example of a molecular solid?
(Multiple Choice)
4.8/5  (42)
(42)
In what type of unit cell are the "A" atoms arranged in the given unit cell? 2 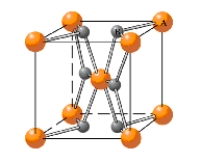
(Multiple Choice)
4.8/5  (40)
(40)
The space-filling representation provided below is an example of a _____ unit cell,which contains _____ atom(s). 
(Multiple Choice)
4.9/5  (40)
(40)
A metal crystallizes in a face-centered cubic lattice.The atomic radius of the metal is 198 pm and the density of the metal is 6.57 g/cm3.What is the volume of the unit cell?
(Multiple Choice)
4.8/5  (42)
(42)
Given the accompanying phase diagram,under what conditions will liquid be found in equilibrium with either solid or gas? 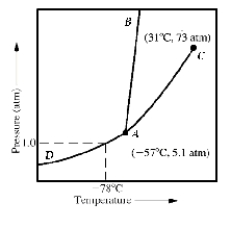
(Multiple Choice)
4.9/5  (35)
(35)
An unknown white solid was found to have a melting point of 150oC.It is soluble in water,but it is a poor conductor of electricity in an aqueous solution.The white solid most likely is _____.
(Multiple Choice)
4.8/5  (32)
(32)
Palladium crystallizes in a face-centered cubic lattice with an edge length of 388.8 pm.What is the density of palladium?
(Multiple Choice)
4.9/5  (48)
(48)
Chromium (atomic mass 52.00 g/mol)crystallizes in a body-centered cubic unit cell.If the length of an edge of the unit cell is 289 pm,what is the density (in g/cm3)of chromium?
(Multiple Choice)
4.8/5  (46)
(46)
Silver chloride adopts the sodium chloride (rock salt)structure.The length of a unit cell edge is 555 pm.What is the density of AgCl?
(Multiple Choice)
4.9/5  (38)
(38)
The energy of formation of one mole of solid crystalline ionic compound when ions in the gas phase combine is referred to as _____.
(Multiple Choice)
4.7/5  (35)
(35)
A metal crystallizes in a face-centered cubic lattice.The radius of the atom is 214 pm and the density of the element is 2.63 g/cm3.What is the identity of the metal?
(Multiple Choice)
4.7/5  (38)
(38)
If a metal crystallizes in a body-centered cubic lattice,each metal atom has _____ "nearest neighbors."
(Multiple Choice)
4.8/5  (39)
(39)
What is the distance,in atomic radii,along any edge of a body-centered cubic unit cell?
(Multiple Choice)
4.7/5  (46)
(46)
Iron(II)sulfide has a primitive cubic unit cell with sulfide ions at the lattice points.The ionic radii of iron(II)ions and sulfide ions are 88 pm and 184 pm,respectively.What is the density of FeS (in g/cm3)?
(Multiple Choice)
4.9/5  (36)
(36)
Which two of the following materials are most likely to be amorphous solids: ice,nylon,glass,potassium nitrate?
(Multiple Choice)
4.8/5  (44)
(44)
The metal vanadium crystallizes in a body-centered cubic lattice.If the density of vanadium is 6.11 g/cm3,what is the unit cell volume?
(Multiple Choice)
4.8/5  (31)
(31)
What is the simplest formula of the compound represented by the unit cell provided below? 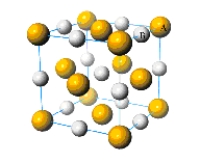
(Multiple Choice)
4.7/5  (40)
(40)
According to the below phase diagram,what process occurs if a pure substance begins at point C and the pressure on the substance is increased until point B is reached? 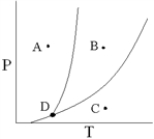
(Multiple Choice)
4.8/5  (32)
(32)
Copper crystallizes in a face-centered cubic lattice.The radius of a copper atom is 128 pm.What is the edge length of the unit cell?
(Multiple Choice)
4.9/5  (42)
(42)
Showing 21 - 40 of 62
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)