Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
How many grams of dioxygen are required to completely burn 4.7 g of C2H5OH?
(Multiple Choice)
4.9/5  (37)
(37)
Potassium hydrogen carbonate decomposes according to the reaction below: 2 KHCO3(s)→ K2CO3(s)+ CO2(g)+ H2O(l)
How many moles of potassium carbonate are produced if 421 g of potassium hydrogen carbonate is heated?
(Multiple Choice)
4.8/5  (32)
(32)
If 5.00 g Br2 and 1.10 g NH3 react according to the equation below,what is the maximum mass of ammonium bromide produced? 3 Br2( 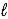 )+ 8 NH3(g)→ 6 NH4Br(s)+ N2(g)
)+ 8 NH3(g)→ 6 NH4Br(s)+ N2(g)
(Multiple Choice)
4.7/5  (43)
(43)
Chlorine was passed over 1.06 g of heated titanium,and 3.40 g of a chloride-containing compound of titanium was obtained.What is the empirical formula of the obtained compound?
(Multiple Choice)
4.8/5  (43)
(43)
What mass of iron can be produced from the reaction of 175 kg Fe2O3 with 385 kg CO? Fe2O3(s)+ 3 CO(g)→ 2 Fe(s)+ 3 CO2(g)
(Multiple Choice)
4.8/5  (37)
(37)
A dilute solution is prepared by transferring 35.00 mL of a 0.6363 M stock solution to a 900.0 mL volumetric flask and diluting to mark.What is the molarity of this dilute solution?
(Multiple Choice)
4.8/5  (35)
(35)
Soft drink bottles are made of polyethylene terephthalate (PET),a polymer composed of carbon,hydrogen,and oxygen.If 1.9022 g PET is burned in oxygen it produces 0.6585 g H2O and 4.0216 g CO2.What is the empirical formula of PET?
(Multiple Choice)
4.8/5  (44)
(44)
Nitric oxide is made from the oxidation of ammonia.What mass of nitric oxide can be made from the reaction of 8.00 g NH3 with 17.0 g O2? 4 NH3(g)+ 5 O2(g)→ 4 NO(g)+ 6 H2O(g)
(Multiple Choice)
4.8/5  (35)
(35)
What volume of 1.27 M HCl is required to prepare 197.4 mL of 0.456 M HCl?
(Multiple Choice)
4.9/5  (34)
(34)
The reaction of 5.07 g N2 with 0.722 g H2 produces 1.27 g NH3.The percent yield of this reaction is ________.
(Short Answer)
4.7/5  (32)
(32)
Zn reacts with hydrochloric acid. Zn(s)+ 2 HCl(aq)→ ZnCl2(aq)+ H2(g)
What volume of 3.05 M HCl(aq)will react with 25.0 g Zn(s)?
(Multiple Choice)
4.8/5  (36)
(36)
An impure sample of benzoic acid (C6H5COOH,122.12 g/mol)is titrated with 0.5360 M NaOH.A 1.888-g sample requires 20.50 mL of titrant to reach the endpoint.What is the percent by mass of benzoic acid in the sample? C6H5COOH(aq)+ NaOH(aq)→ NaC6H5COO(aq)+ H2O(l)
(Multiple Choice)
4.8/5  (41)
(41)
In order to dilute 77.1 mL of 0.778 M HCl to 0.100 M,the volume of water that must be added is
(Multiple Choice)
4.9/5  (38)
(38)
An aqueous nitric acid solution has a pH of 2.15.What mass of nitric acid,HNO3,is present in 20.0 L of this solution?
(Multiple Choice)
4.8/5  (41)
(41)
Polyethylene is a polymer consisting of only carbon and hydrogen.If 2.300 g of the polymer is burned in oxygen it produces 2.955 g of H2O and 7.217 g of CO2.Determine the empirical formula of polyethylene.
(Multiple Choice)
4.8/5  (40)
(40)
A 1.295 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 3.553 g CO2 and water.What mass of hydrogen is contained in the original sample?
(Multiple Choice)
4.9/5  (41)
(41)
Consider the fermentation reaction of glucose: C6H12O6 → 2C2H5OH + 2CO2
A 1)00-mol sample of C6H12O6 was placed in a vat with 100 g of yeast.If 38.5 g of C2H5OH was obtained,what was the percent yield of C2H5OH?
(Multiple Choice)
4.8/5  (35)
(35)
The reaction of 0.779 g K with O2 forms 1.417 g potassium superoxide,a substance used in self-contained breathing devices.Determine the formula for potassium superoxide.
(Multiple Choice)
4.9/5  (40)
(40)
Showing 21 - 40 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)