Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
How many sulfur dioxide molecules are there in 1.80 mol of sulfur dioxide?
(Multiple Choice)
4.8/5  (30)
(30)
When the following equation is balanced, the coefficient of O2 is _ . 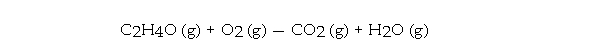
(Multiple Choice)
4.7/5  (42)
(42)
A nitrogen oxide is 63.65% by mass nitrogen. The molecular formula could be _ _.
(Multiple Choice)
4.8/5  (33)
(33)
Calculate the percentage by mass of hydrogen in PtCl2(NH3)2.
(Multiple Choice)
5.0/5  (30)
(30)
A great deal of the carbon dioxide produced by the combustion of fossil fuels is absorbed into the oceans.
(True/False)
4.9/5  (31)
(31)
Which one of the following is not true concerning automotive air bags?
(Multiple Choice)
4.8/5  (32)
(32)
A compound that is composed of only carbon and hydrogen contains 85.7% C and 14.3% H by mass. What is the empirical formula of the compound?
(Multiple Choice)
4.9/5  (33)
(33)
The combustion of propane (C3H8) produces CO2 and H2O:
 The reaction of 2.5 mol of O2 will produce mol of H2O.
The reaction of 2.5 mol of O2 will produce mol of H2O.
(Multiple Choice)
4.8/5  (29)
(29)
Calculate the percentage by mass of chlorine in PtCl2(NH3)2.
(Multiple Choice)
4.8/5  (40)
(40)
How many moles of carbon dioxide are there in 52.06 g of carbon dioxide?
(Multiple Choice)
4.8/5  (25)
(25)
What is the maximum mass in grams of NH3 that can be produced by the reaction of 1.0 g of N2 with 3.0 g of H2 via the equation below? 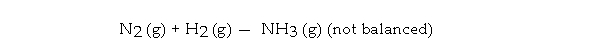
(Multiple Choice)
4.9/5  (38)
(38)
Under appropriate conditions, nitrogen and hydrogen undergo a combination reaction to yield ammonia:
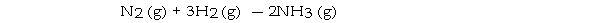 A 9.3- g sample of hydrogen requires
A 9.3- g sample of hydrogen requires
(Essay)
4.8/5  (28)
(28)
What mass in grams of hydrogen is produced by the reaction of 4.73 g of magnesium with 1.83 g of water? 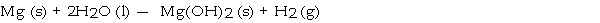
(Multiple Choice)
4.9/5  (29)
(29)
Of the reactions below, which one is a decomposition reaction?
(Multiple Choice)
4.9/5  (42)
(42)
A compound contains 40.0% C, 6.71% H, and 53.29% O by mass. The molecular weight of the compound is 60.05 amu. The molecular formula of this compound is _ _.
(Multiple Choice)
4.8/5  (40)
(40)
The combustion of carbon disulfide in the presence of excess oxygen yields carbon dioxide and sulfur dioxide:
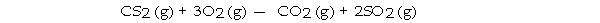 The combustion of 15 g of CS2 in the presence of excess oxygen yields g of SO2.
The combustion of 15 g of CS2 in the presence of excess oxygen yields g of SO2.
(Short Answer)
4.9/5  (40)
(40)
Carbon dioxide called a greenhouse gas because bacterial degradation of fertilizers in a greenhouse environment produce large quantities of carbon dioxide.
(True/False)
4.8/5  (41)
(41)
A compound was found to contain 90.6% lead (Pb) and 9.4% oxygen. The empirical formula for this compound is .
(Short Answer)
4.8/5  (28)
(28)
Showing 61 - 80 of 134
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)