Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
GeF3H is formed from GeH4 and GeF4 in the combination reaction:
 If the reaction yield is 92.6%, how many moles of GeF4 are needed to produce 8.00 mol of GeF3H?
If the reaction yield is 92.6%, how many moles of GeF4 are needed to produce 8.00 mol of GeF3H?
(Multiple Choice)
4.8/5  (38)
(38)
Water can be formed from the stoichiometric reaction of hydrogen with oxygen:
 A complete reaction of 5.0 g of O2 with excess hydrogen produces g of H2O.
A complete reaction of 5.0 g of O2 with excess hydrogen produces g of H2O.
(Short Answer)
4.9/5  (29)
(29)
Sulfur and fluorine react in a combination reaction to produce sulfur hexafluoride:
 In a particular experiment, the percent yield is 79.0%. This means that a 7.90- g sample of fluorine yields g of SF6 in the presence of excess sulfur.
In a particular experiment, the percent yield is 79.0%. This means that a 7.90- g sample of fluorine yields g of SF6 in the presence of excess sulfur.
(Multiple Choice)
4.9/5  (34)
(34)
When the following equation is balanced, the coefficient of H2S is . 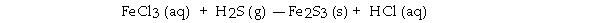
(Multiple Choice)
4.7/5  (34)
(34)
The mass of a single atom of an element (in amu) is numerically EQUAL to the mass in grams of 1 mole of that element.
(True/False)
4.8/5  (39)
(39)
Lithium and nitrogen react in a combination reaction to produce lithium nitride:
6Li (s) + N2 (g) -2Li3N (s)
How many moles of lithium nitride are produced when 0.450 mol of lithium react in this fashion?
(Multiple Choice)
4.8/5  (36)
(36)
When the following equation is balanced, the coefficient of water is _. 
(Multiple Choice)
4.9/5  (35)
(35)
Silver nitrate and aluminum chloride react with each other by exchanging anions:
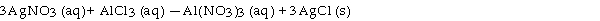 What mass in grams of AgCl is produced when 4.22 g of AgNO3 react with 7.73 g of AlCl3?
What mass in grams of AgCl is produced when 4.22 g of AgNO3 react with 7.73 g of AlCl3?
(Multiple Choice)
4.9/5  (34)
(34)
When a hydrocarbon burns in air, what component of air reacts?
(Multiple Choice)
4.7/5  (34)
(34)
When the following equation is balanced, the coefficient of sulfur dioxide is . 
(Multiple Choice)
5.0/5  (31)
(31)
Combustion of a 0.9835- g sample of a compound containing only carbon, hydrogen, and oxygen produced 1.900 g of CO2 and 1.070 g of H2O. What is the empirical formula of the compound?
(Multiple Choice)
4.8/5  (30)
(30)
What is the mass in grams of 9.76 × 1012 atoms of naturally occurring sodium?
(Multiple Choice)
4.8/5  (31)
(31)
There are molecules of methane in 0.123 mol of methane (CH4).
(Multiple Choice)
4.9/5  (25)
(25)
What is the empirical formula of a compound that contains 27.0% S, 13.4% O, and 59.6% Cl by mass?
(Multiple Choice)
4.8/5  (38)
(38)
Gaseous argon has a density of 1.40 g/L at standard conditions. How many argon atoms are in 1.00 L of argon gas at standard conditions?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 81 - 100 of 134
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)