Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Under appropriate conditions, nitrogen and hydrogen undergo a combination reaction to yield ammonia:
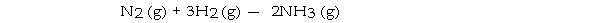 A 7.1- g sample of N2 requires g of H2 for complete reaction.
A 7.1- g sample of N2 requires g of H2 for complete reaction.
(Multiple Choice)
4.9/5  (26)
(26)
Which one of the following substances is the product of this combination reaction?
Al (s) + I2 (s) -
(Multiple Choice)
4.9/5  (37)
(37)
When the following equation is balanced, the coefficient of water is _. 
(Multiple Choice)
4.9/5  (28)
(28)
The combustion of propane (C3H8) in the presence of excess oxygen yields CO2 and H2O:
 When 2.5 mol of O2 are consumed in their reaction, mol of CO2 are produced.
When 2.5 mol of O2 are consumed in their reaction, mol of CO2 are produced.
(Multiple Choice)
4.8/5  (36)
(36)
A sample of C3H8O that contains 200 molecules contains _ carbon atoms.
(Multiple Choice)
4.8/5  (30)
(30)
Of the reactions below, which one is not a combination reaction?
(Multiple Choice)
4.9/5  (36)
(36)
A compound is composed of only C, H, and O. The combustion of a 0.519- g sample of the compound yields 1.24 g of CO2 and 0.255 g of H2O. What is the empirical formula of the compound?
(Multiple Choice)
4.9/5  (38)
(38)
Complete and balance the following reaction, given that elemental rubidium reacts with elemental sulfur to form Rb2S (s).
Na (s) + S (s) -
(Short Answer)
4.9/5  (34)
(34)
The quantity of product that is calculated to form when all of the limiting reagent reacts is called the actual yield.
(True/False)
4.8/5  (32)
(32)
When the following equation is balanced, the coefficient of H2 is _ . 
(Multiple Choice)
4.8/5  (34)
(34)
The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O:
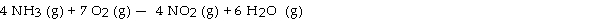 The combustion of 43.9 g of ammonia produces g of NO2.
The combustion of 43.9 g of ammonia produces g of NO2.
(Multiple Choice)
4.8/5  (40)
(40)
Combustion of a 1.031- g sample of a compound containing only carbon, hydrogen, and oxygen produced 2.265 g of CO2 and 1.236 g of H2O. What is the empirical formula of the compound?
(Multiple Choice)
4.9/5  (40)
(40)
When the following equation is balanced, the coefficient of dinitrogen pentoxide is . 
(Multiple Choice)
4.9/5  (38)
(38)
When the following equation is balanced, the coefficient of Al is . 
(Multiple Choice)
4.7/5  (40)
(40)
When the following equation is balanced, the coefficient of H2O is _ . 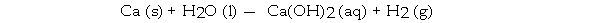
(Multiple Choice)
4.8/5  (34)
(34)
When the following equation is balanced, the coefficient of Al2O3 is _ . 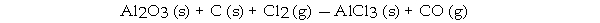
(Multiple Choice)
4.8/5  (29)
(29)
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
 In a particular experiment, a 5.00- g sample of CaO is reacted with excess water and 6.11 g of Ca(OH)2 is recovered. What is the percent yield in this experiment?
In a particular experiment, a 5.00- g sample of CaO is reacted with excess water and 6.11 g of Ca(OH)2 is recovered. What is the percent yield in this experiment?
(Multiple Choice)
4.9/5  (28)
(28)
Showing 21 - 40 of 134
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)