Exam 2: Basic Chemistry
Exam 1: A View of Life52 Questions
Exam 2: Basic Chemistry54 Questions
Exam 3: The Chemistry of Organic Molecules55 Questions
Exam 4: Cell Structure and Function55 Questions
Exam 5: Membrane Structure and Function60 Questions
Exam 6: Metabolism: Energy and Enzymes54 Questions
Exam 7: Photosynthesis51 Questions
Exam 8: Cellular Respiration49 Questions
Exam 9: The Cell Cycle and Cellular Reproduction57 Questions
Exam 10: Meiosis and Sexual Reproduction61 Questions
Exam 11: Mendelian Patterns of Inheritance61 Questions
Exam 12: Molecular Biology of the Gene53 Questions
Exam 13: Regulation of Gene Expression49 Questions
Exam 14: Biotechnology and Genomics52 Questions
Exam 15: Darwin and Evolution59 Questions
Exam 16: How Populations Evolve55 Questions
Exam 17: Speciation and Macroevolution55 Questions
Exam 18: Origin and History of Life57 Questions
Exam 19: Taxonomy,systematics,and Phylogeny53 Questions
Exam 20: Viruses,bacteria,and Archaea59 Questions
Exam 21: Protist Evolution and Diversity46 Questions
Exam 22: Fungi Evolution and Diversity53 Questions
Exam 23: Plant Evolution and Diversity63 Questions
Exam 24: Flowering Plants: Structure and Organization63 Questions
Exam 25: Flowering Plants: Nutrition and Transport56 Questions
Exam 26: Flowering Plants: Control of Growth Responses52 Questions
Exam 27: Flowering Plants: Reproduction52 Questions
Exam 28: Invertebrate Evolution53 Questions
Exam 29: Vertebrate Evolution57 Questions
Exam 30: Human Evolution51 Questions
Exam 31: Animal Organization and Homeostasis51 Questions
Exam 32: Circulation and Cardiovascular Systems57 Questions
Exam 33: The Lymphatic and Immune Systems55 Questions
Exam 34: Digestive Systems and Nutrition57 Questions
Exam 35: Respiratory Systems53 Questions
Exam 36: Body Fluid Regulation and Excretory Systems53 Questions
Exam 37: Neurons and Nervous Systems55 Questions
Exam 38: Sense Organs57 Questions
Exam 39: Locomotion and Support Systems55 Questions
Exam 40: Hormones and Endocrine Systems52 Questions
Exam 41: Reproductive Systems58 Questions
Exam 42: Animal Development and Aging53 Questions
Exam 43: Behavioral Ecology51 Questions
Exam 44: Population Ecology49 Questions
Exam 45: Community and Ecosystem Ecology55 Questions
Exam 46: Major Ecosystems of the Biosphere58 Questions
Exam 47: Conservation of Biodiversity46 Questions
Select questions type
A solution with a pH of 6 has ________ times ________ OH- than a solution with a pH of 10.
Free
(Multiple Choice)
4.7/5  (29)
(29)
Correct Answer:
C
Which one is NOT one of the properties of water?
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
A
Draw three water molecules and the hydrogen bonding that may occur between the molecules.Define hydrogen bonding and explain how and why it occurs.
Free
(Essay)
4.9/5  (26)
(26)
Correct Answer:
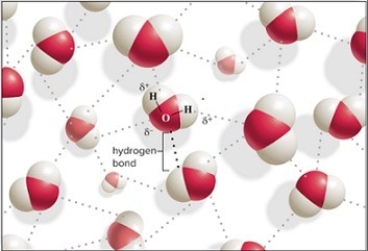 The hydrogen bonding is shown as dotted lines between the water molecules.Hydrogen bonding is the weak attraction between a covalently bonded hydrogen atom and an electronegative atom taking part in another covalent bond.It occurs between a partially positive hydrogen in one water molecule and a partially negative oxygen in another water molecule.The hydrogen has a partially positive charge and the oxygen has a partially negative charge because of the unequal sharing of electrons.
The hydrogen bonding is shown as dotted lines between the water molecules.Hydrogen bonding is the weak attraction between a covalently bonded hydrogen atom and an electronegative atom taking part in another covalent bond.It occurs between a partially positive hydrogen in one water molecule and a partially negative oxygen in another water molecule.The hydrogen has a partially positive charge and the oxygen has a partially negative charge because of the unequal sharing of electrons.
Which of the following statements is/are true about the pH scale?
-The scale indicates the relative concentrations of hydrogen and hydroxide ions in a solution.
(True/False)
4.8/5  (33)
(33)
Which of the following statements is/are true about the pH scale?
-pH 7 has a balanced level of H+ and OH-.
(True/False)
4.8/5  (38)
(38)
Which of the following statements is NOT true about electron configurations?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following statements is/are true about the pH scale?
-Any substance below pH 7 is considered acidic and any substance above pH 7 is considered basic or alkaline.
(True/False)
4.9/5  (29)
(29)
Draw two hydrogen atoms using Bohr's model.Now bond them to form a molecule of hydrogen gas.Write the molecular formula.Explain what type of bond you've created and why this is a stable situation.
(Essay)
4.8/5  (26)
(26)
Which property of water allows it stick to surfaces like glass?
(Multiple Choice)
4.8/5  (26)
(26)
Both 18O and 16O are found in nature.However,16O is the most common.Therefore,
(Multiple Choice)
4.9/5  (32)
(32)
Draw the structural formula of a single water molecule.Note the location of partial positive and negative charges.Label the covalent bonds.
(Essay)
4.7/5  (40)
(40)
Study the chart to determine the relationship between H+ concentration and pH.If you were to create a herbal remedy to decrease excess stomach acid,would you create a solution with a relatively greater or lesser number of hydrogen ions? (moles per liter) 0.000001=1\times1 6 0.0000001=1\times1 7 0.00000001=1\times1 8
(Essay)
4.8/5  (37)
(37)
This system of chemicals, ,act as a buffer in the blood.If hydrogen ions are added to blood which of the following reactions would occur?
(Multiple Choice)
5.0/5  (45)
(45)
Draw several (5 or 6)individual,unbonded water molecules.Simulate what happens when table salt (Na+Cl-)is added to water.Use the model you created to explain why salt is added to the roads in a "snowy," cold climate.
(Essay)
4.8/5  (33)
(33)
The periodic table displays the following information about the element phosphorus: 15
P
30.794
Based on this information,which of the following are true statements about phosphorus? Select all that apply.
(Multiple Choice)
4.7/5  (34)
(34)
Following nitrogen (78%)and oxygen (21%),argon is the next most common gas in the atmosphere (less than 1%).Checking the table of elements,you discover that argon is one of a family of atoms with outer shells already full of electrons.How is this related to the fact that these atoms have virtually no biological importance?
(Essay)
4.7/5  (31)
(31)
An article in a medical journal indicates that researchers have used an isotope 3H to trace a certain metabolic process.From the symbol that is given,we know this is a hydrogen isotope with
(Multiple Choice)
4.7/5  (33)
(33)
Showing 1 - 20 of 54
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)