Exam 5: Chemical Reactions
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
Ammonia and sulfuric acid react according to the equation given below. How many milliliters of 0.110 M sulfuric acid are required to exactly neutralize 25.0 mL of 0.0840 M NH3 solution?
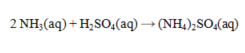
(Multiple Choice)
4.9/5  (29)
(29)
If a 45.0 mL sample of 2.20 M Na2SO4 is diluted to yield a final solution that is 0.110 M in sodium ions, what is the volume of the final solution?
(Multiple Choice)
4.8/5  (42)
(42)
A chemical reaction for which spectator ions are deleted is called a(n) _____________ ionic equation.
(Short Answer)
4.9/5  (41)
(41)
Which compound will not dissolve in water in large amounts?
(Multiple Choice)
4.8/5  (29)
(29)
A solution is made by dissolving 60.0 g of AlCl3 in enough water to make 250.0 mL of solution. How many moles of ions are in 5.00 mL of solution?
(Multiple Choice)
5.0/5  (34)
(34)
A 25.00 mL sample of HCl solution is neutralized by exactly 41.63 mL of 0.1363 M NaOH. What is the molarity of the HCl solution?
(Multiple Choice)
4.9/5  (40)
(40)
Which statement about the reaction below is true, given large amounts of reactants? 
(Multiple Choice)
4.9/5  (39)
(39)
How many grams of PbI2 will precipitate from the reaction of 14.0 mL of 0.190 M Pb(NO3)2 with excess KI solution? Assume that all of the PbI2 is insoluble.
(Multiple Choice)
4.9/5  (27)
(27)
What is the net ionic equation for the reaction of AlCl3 and NaOH?
(Multiple Choice)
4.9/5  (32)
(32)
What is the net ionic equation for the reaction of Ca(NO3)2 and Na2SO4?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 41 - 60 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)