Exam 15: Acids and Bases
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
Which pair of substances does not represent a conjugate acid-base pair?
(Multiple Choice)
4.8/5  (34)
(34)
A wine sample had a pH of 3.52. This corresponds to [H3O+] =
(Multiple Choice)
4.8/5  (38)
(38)
In terms of base strength, which base does not belong in a category with the others?
(Multiple Choice)
4.8/5  (23)
(23)
The product of the reaction between methylamine, CH3NH2, and hydrochloric acid are
(Multiple Choice)
4.8/5  (42)
(42)
In a 1.2 M solution of KOH, a strong base, [H3O+] = ____, and [OH-] = ____.
(Multiple Choice)
4.7/5  (30)
(30)
Which substance is a Lewis acid but not a Bronsted-Lowry acid?
(Multiple Choice)
4.8/5  (33)
(33)
Which factor does not contribute to the acidity of the hydrogen atom in the carboxylic acid functional group?
(Multiple Choice)
4.9/5  (31)
(31)
Calculate the pH of a 0.051 M solution of sodium lactate. The Ka for lactic acid is 1.4 × 10-4.
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following salts forms a basic solution when dissolved in water?
(Multiple Choice)
4.8/5  (39)
(39)
Which substance can act as a Bronsted-Lowry base, but not as a Bronsted-Lowry acid?
(Multiple Choice)
4.9/5  (33)
(33)
Which salt forms a neutral solution when dissolved in water?
(Multiple Choice)
4.8/5  (39)
(39)
Because water can act as a Bronsted-Lowry acid or base, it is said to be ____.
(Multiple Choice)
4.8/5  (45)
(45)
Classify each compound as a carboxylic acid or an amine, in the order given. 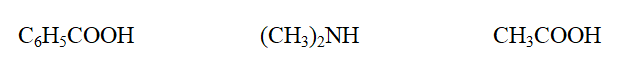
(Multiple Choice)
4.9/5  (41)
(41)
Showing 21 - 40 of 62
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)