Exam 15: Acids and Bases
Exam 1: The Nature of Chemistry68 Questions
Exam 2: Atoms and Elements66 Questions
Exam 3: Chemical Compounds65 Questions
Exam 4: Quantities of Reactants and Products65 Questions
Exam 5: Chemical Reactions66 Questions
Exam 6: Energy and Chemical Reactions55 Questions
Exam 7: Electron Configurations and the Periodic Table64 Questions
Exam 8: Covalent Bonding67 Questions
Exam 9: Molecular Structure53 Questions
Exam 10: Gases and the Atmosphere57 Questions
Exam 11: Liquids, Solids, and Materials46 Questions
Exam 12: Chemical Kinetics: Rates of Reactions66 Questions
Exam 13: Chemical Equilibrium57 Questions
Exam 14: The Chemistry of Solutes and Solutions57 Questions
Exam 15: Acids and Bases62 Questions
Exam 16: Additional Aqueous Equilibria52 Questions
Exam 17: Thermodynamics: Directionality of Chemical Reactions56 Questions
Exam 18: Electrochemistry and Its Applications54 Questions
Exam 19: Nuclear Chemistry53 Questions
Select questions type
In an acidic solution at 25 C, which of the following is not true?
(Multiple Choice)
4.8/5  (39)
(39)
The acid ionization constant expression for the ionization of the hydrogen sulfate ion, 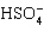 , in aqueous solution is
, in aqueous solution is
(Multiple Choice)
4.8/5  (38)
(38)
In a 1.2 M solution of HClO4, a strong acid, [H3O+] = ____, and [OH-] = ____.
(Multiple Choice)
4.9/5  (37)
(37)
Identify which of the hydrohalic acids is unlike the others, and why.
(Multiple Choice)
4.9/5  (31)
(31)
Ammonia is a weak base and perchloric acid is a strong acid. Which statement is true of a solution of ammonium perchlorate?
(Multiple Choice)
4.8/5  (37)
(37)
Ammonia is a weak base and acetic acid is a weak acid. Which statement is true of a solution of ammonium acetate?
(Multiple Choice)
4.9/5  (35)
(35)
Which pair of substances represents a conjugate acid-base pair?
(Multiple Choice)
4.9/5  (29)
(29)
In a neutral polyprotic acid, the value of Ka2 will be ____ the value of Ka1 because ____.
(Multiple Choice)
4.9/5  (33)
(33)
A solution is not neutral. Which one of these statements is true?
(Multiple Choice)
4.9/5  (35)
(35)
Methylamine, CH3NH2, acts as a weak base in water. The products of the reaction are ____ and ____.
(Multiple Choice)
4.9/5  (40)
(40)
One water molecule can donate a proton to another in a process called ____; the equilibrium constant expression for this reaction is ____.
(Multiple Choice)
4.8/5  (31)
(31)
If the hydrogen ion concentration of a solution is increased by a factor of 4, the pH is
(Multiple Choice)
4.7/5  (42)
(42)
A solution of acetic acid (Ka = 1.8 × 10-5) has a pH exactly 2 higher than a solution of HCl(aq) of the same concentration. What is that concentration?
(Essay)
4.8/5  (39)
(39)
Showing 41 - 60 of 62
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)