Exam 5: Thermochemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Which of the following is a statement of the first law of thermodynamics?
(Multiple Choice)
4.8/5  (36)
(36)
Given the data in the table below,ΔH°rxn for the reaction C2H5OH (l)+ O2 (g)→ CH3CO2H (l)+ H2O (l)
Is ________ kJ. 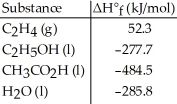
(Multiple Choice)
4.7/5  (31)
(31)
Given the data in the table below,ΔH°rxn for the reaction Ca(OH)2 + 2H3AsO4 → Ca(H2AsO4)2 + 2H2O
Is ________ kJ. 
(Multiple Choice)
4.9/5  (29)
(29)
How many kJ of heat are released when 15.75 g of Ba (s)reacts completely with oxygen gas to form BaO (s)? ΔH° = -1107 kJ.
(Multiple Choice)
4.8/5  (28)
(28)
Hydrogen peroxide decomposes to water and oxygen at constant pressure (△H = -196 kJ). What is the value of q (kJ)for this reaction when 4.60 g of hydrogen peroxide decomposes at constant pressure?
(Multiple Choice)
4.8/5  (32)
(32)
ΔH for the reaction IF5 (g)→ IF3 (g)+ F2 (g)
Is ________ kJ,give the data below.
IF (g)+ F2 (g)→ IF3 (g)ΔH = -390 kJ
IF (g)+ 2F2 (g)→ IF5 (g)ΔH = -745 kJ
(Multiple Choice)
4.9/5  (35)
(35)
Coal contains hydrocarbons of high molecular weight as well as compounds containing ________,sulfur,or nitrogen.
(Short Answer)
4.7/5  (32)
(32)
An 8 oz.bottle of energy drink contains 6.0 g of protein,2.0 g of fat,and 16.3 g of carbohydrate.The fuel value of this energy drink bottle is ________ kJ.The fuel values for protein,fat,and carbohydrate are 17,38,and 17 kJ/g,respectively.
(Multiple Choice)
4.9/5  (34)
(34)
The ΔHrxn for the combustion of methane is -890.0 kJ.How much heat energy (kJ)is released if 82.1 grams of methane are burned in an excess amount of oxygen?
(Short Answer)
4.9/5  (34)
(34)
The specific heat capacity of liquid water is 4.18 J/g-K.How many joules of heat are needed to raise the temperature of 7.25 g of water from 20.0 °C to 44.1 °C?
(Multiple Choice)
4.8/5  (39)
(39)
The specific heat capacity of lead is 0.13 J/g-K.How much heat (in J)is required to raise the temperature of 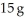 of lead from 22 °C to 37 °C?
of lead from 22 °C to 37 °C?
(Multiple Choice)
4.8/5  (30)
(30)
The value of ΔH° for the reaction below is -186 kJ. H2 (g)+ Cl2 (g)→ 2HCl (g)
The value of ΔH°f for HCl (g)is ________ kJ/mol.
(Multiple Choice)
4.8/5  (30)
(30)
Given the following reactions N2 (g)+ O2 (g)→ 2NO (g)ΔH = +180.7 kJ
2NO( g)+ O2 (g)→ 2NO2 (g)ΔH = -113.1 kJ
The enthalpy for the decomposition of nitrogen dioxide into molecular nitrogen and oxygen
2NO2 (g)→ N2 (g)+ 2O2 (g)
Is ________ kJ.
(Multiple Choice)
4.8/5  (29)
(29)
Carbon monoxide and oxygen gas react to form carbon dioxide.How much heat is released when 89.5 grams of O2 (g)reacts with excess CO? ΔH° = -482 kJ.
(Multiple Choice)
5.0/5  (24)
(24)
A 19.5 g candy bar contains 8% protein,33% fat,and 18% carbohydrate.The respective fuel values for protein,fat,and carbohydrate are 17,38,and 17 kJ/g,respectively.What is the fuel value (kJ)for this piece of candy?
(Multiple Choice)
4.8/5  (28)
(28)
The value of ΔE for a system that performs 151 kJ of work on its surroundings and loses 79 kJ of heat is ________ kJ.
(Multiple Choice)
4.9/5  (34)
(34)
The internal energy can be increased by ________. (a)transferring heat from the surroundings to the system
(b)transferring heat from the system to the surroundings
(c)doing work on the system
(Multiple Choice)
4.8/5  (28)
(28)
Showing 121 - 140 of 154
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)