Exam 5: Thermochemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
What is the kinetic energy of a 145 g baseball traveling at 89.9 mi/hr? 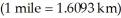
(Multiple Choice)
4.7/5  (28)
(28)
An 6.11 g sample of calcium carbonate [CaCO3 (s)] absorbs ![An 6.11 g sample of calcium carbonate [CaCO<sub>3</sub> (s)] absorbs of heat,upon which the temperature of the sample increases from to What is the specific heat of calcium carbonate?](https://storage.examlex.com/TB1194/11ea7e7c_6114_aa84_9a0a_070dcb9f99a9_TB1194_11.jpg) of heat,upon which the temperature of the sample increases from
of heat,upon which the temperature of the sample increases from ![An 6.11 g sample of calcium carbonate [CaCO<sub>3</sub> (s)] absorbs of heat,upon which the temperature of the sample increases from to What is the specific heat of calcium carbonate?](https://storage.examlex.com/TB1194/11ea7e7c_6114_aa85_9a0a_b34b89508b2f_TB1194_11.jpg) to
to ![An 6.11 g sample of calcium carbonate [CaCO<sub>3</sub> (s)] absorbs of heat,upon which the temperature of the sample increases from to What is the specific heat of calcium carbonate?](https://storage.examlex.com/TB1194/11ea7e7c_6114_aa86_9a0a_cd0e2f4cc7b2_TB1194_11.jpg) What is the specific heat of calcium carbonate?
What is the specific heat of calcium carbonate?
(Multiple Choice)
4.9/5  (35)
(35)
Given the data in the table below,ΔH°rxn for the reaction 2Ag2S (s)+ O2 (g)→ 2Ag2O (s)+ 2S (s)
Is ________ kJ. 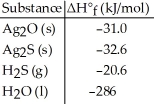
(Multiple Choice)
4.9/5  (36)
(36)
Calculate the kinetic energy in joules of a 150 lb jogger (68.1 kg)traveling at 12.0 mile/hr (5.36 m/s).
(Multiple Choice)
4.9/5  (35)
(35)
The value of ΔH° for the following reaction is -3351 kJ: 2Al (s)+ 3O2(g)→ 2Al2O3(s)
The value of ΔH°f for Al2
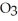 (s)is ________ kJ.
(s)is ________ kJ.
(Multiple Choice)
4.8/5  (36)
(36)
For the combustion reaction of methane,ΔH°f is zero for ________. CH4 (g)+ O2 (g)→ 2H2O(g)+ CO2 (g)
(Multiple Choice)
4.7/5  (23)
(23)
The ΔE of a system that releases 12.4 J of heat and does 4.2 J of work on the surroundings is ________ J.
(Multiple Choice)
4.9/5  (45)
(45)
The temperature of a 35.1 g sample of iron increases from 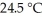 to
to 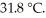 If the specific heat of iron is
If the specific heat of iron is 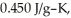 how many joules of heat are absorbed?
how many joules of heat are absorbed?
(Multiple Choice)
4.8/5  (37)
(37)
A 3.00 L pitcher of sweetened ice tea contains 600.g of sugar.Assuming that the sugar is the only fuel source,what is the fuel value (in kJ)of a 250.mL serving? The respective fuel values for protein,fat,and carbohydrate are 17,38,and 17 kJ/g,respectively.
(Multiple Choice)
4.8/5  (39)
(39)
At what velocity (m/s)must a 417.3 g object be moving in order to possess a kinetic energy of 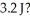
(Multiple Choice)
4.8/5  (34)
(34)
Calculate the work (kJ)done during a reaction in which the internal volume expands from 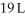 to
to 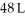 against an outside pressure of
against an outside pressure of 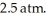
(Multiple Choice)
4.8/5  (44)
(44)
Given the data in the table below,ΔH°rxn for the reaction SO3 (g)+ H2O (l)→ H2SO4 (l)
Is ________ kJ. 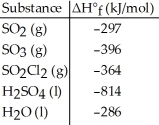
(Multiple Choice)
4.7/5  (35)
(35)
Given the data in the table below,ΔH°rxn for the reaction Ag2O (s)+ H2S (g)→ Ag2S (s)+ H2O (l)
Is ________ kJ. 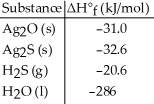
(Multiple Choice)
4.8/5  (36)
(36)
For the following reactions,the ΔH°rxn is NOT equal to ΔH°f for the product except for ________.
(Multiple Choice)
4.9/5  (38)
(38)
For the following reactions,the ΔH°rxn is NOT equal to ΔH°f for the product except for ________.
(Multiple Choice)
4.9/5  (35)
(35)
The kinetic energy of a 10.3 g golf ball traveling at 48.0 m/s is ________ J.
(Multiple Choice)
4.8/5  (30)
(30)
At what velocity (m/s)must a 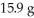 object be moving in order to possess a kinetic energy of
object be moving in order to possess a kinetic energy of 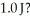
(Multiple Choice)
4.7/5  (34)
(34)
A 50.0-g sample of liquid water at 25.0 °C is mixed with 23.0 g of water at 79.0 °C.The final temperature of the water is ________ °C.
(Multiple Choice)
4.8/5  (36)
(36)
Showing 81 - 100 of 154
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)