Exam 5: Thermochemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
All of the following are considered fossil fuels except ________.
(Multiple Choice)
4.9/5  (34)
(34)
Given the following reactions 2S (s)+ 3O2 (g)→ 2SO3 (g)ΔH = -790 kJ
S (s)+ O2 (g)→ SO2(g)ΔH = -297 kJ
The enthalpy of the reaction in which sulfur dioxide is oxidized to sulfur trioxide
2SO2 (g)+ O2 (g)→ 2SO3 (g)
Is ________ kJ.
(Multiple Choice)
4.9/5  (41)
(41)
Given the following reactions N2 (g)+ 2O2 (g)→ 2NO2 (g)ΔH = 66.4 kJ
2NO (g)+ O2 (g)→ 2NO2 (g)ΔH = -114.2 kJ
The enthalpy of the reaction of the nitrogen to produce nitric oxide
N2 (g)+ O2 (g)→ 2NO (g)
Is ________ kJ.
(Multiple Choice)
4.8/5  (29)
(29)
Given the data in the table below,ΔH°rxn for the reaction 2SO2 (g)+ O2 (g)→ 2SO3 (g)
Is ________ kJ. 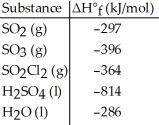
(Multiple Choice)
4.8/5  (34)
(34)
In the presence of excess oxygen,methane gas burns in a constant-pressure system to yield carbon dioxide and water: CH4 (g)+ 2O2 (g)→ CO2 (g)+ 2H2O (l)△H = -890.0 kJ
Calculate the value of q (kJ)in this exothermic reaction when 1.80 g of methane is combusted at constant pressure.
(Multiple Choice)
4.9/5  (34)
(34)
The term Btu which stands for ________ is commonly used in engineering applications.
(Multiple Choice)
4.7/5  (31)
(31)
How much heat is required to raise the temperature of a 1.15 kg piece of copper metal from 25.0 °C to 77.5 °C? The specific heat capacity of solid copper metal is 0.385 J/g-K.
(Multiple Choice)
4.8/5  (26)
(26)
A 100-watt electric incandescent light bulb consumes ________ J of energy in 24 hours.[1 Watt (W)= 1 J/sec]
(Multiple Choice)
4.9/5  (43)
(43)
The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l)→ CO (g)+ 2H2 (g)
How many kJ of heat are consumed when 5.10 g of CO (g)is formed as shown in the equation?
(Multiple Choice)
4.9/5  (32)
(32)
The temperature of a 15-g sample of lead metal increases from 22 °C to 37 °C upon the addition of 29.0 J of heat.The specific heat capacity of the lead is ________ J/g-K.
(Multiple Choice)
5.0/5  (29)
(29)
Given the following reactions N2 (g)+ O2 (g)→ 2NO (g)ΔH = +180.7 kJ
2NO (g)+ O2(g)→ 2N 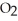 (g)ΔH = -113.1 kJ
The enthalpy of reaction for
4NO (g)→ 2NO2 (g)+ N2 (g)
Is ________ kJ.
(g)ΔH = -113.1 kJ
The enthalpy of reaction for
4NO (g)→ 2NO2 (g)+ N2 (g)
Is ________ kJ.
(Multiple Choice)
4.7/5  (29)
(29)
A typical fast food meal consists of a burger,fries,and a soft-drink and contains 58.0 grams of fat,39.0 grams of protein,and 177 grams of carbohydrate.If jogging burns 950.0 kJ/hour,how many minutes would it take to completely burn off the meal? The respective fuel values for protein,fat,and carbohydrate are 17,38,and 17 kJ/g,respectively.
(Multiple Choice)
4.8/5  (35)
(35)
Given the data in the table below,ΔH°rxn for the reaction IF5 (g)+ F2 (g)→ IF7 (g)
Is ________ kJ. 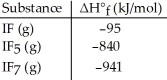
(Multiple Choice)
4.9/5  (36)
(36)
A 26.9 g rock rolls down the hill at a speed of 81.9 m/s .What is the kinetic energy of the rock?
(Multiple Choice)
4.9/5  (25)
(25)
How many joules of heat are absorbed when the temperature of a 13.9 g sample of CaCO3 (s)increases from 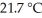 to
to 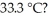 Specific heat of calcium carbonate is
Specific heat of calcium carbonate is 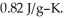
(Multiple Choice)
4.9/5  (32)
(32)
The change in the internal energy of a system that absorbs 2,500 J of heat and that does 7,655 J of work on the surroundings is ________ J.
(Multiple Choice)
4.9/5  (39)
(39)
The value of ΔH° for the following reaction is 177.8 kJ.The value of Δ 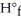 for CaO(s)is ________ kJ/mol. CaCO3 (s)→ CaO (s)+ CO2 (g)
for CaO(s)is ________ kJ/mol. CaCO3 (s)→ CaO (s)+ CO2 (g) 
(Multiple Choice)
4.8/5  (23)
(23)
The temperature of a 24.3 g sample of gold increases from 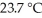 to
to 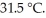 If the specific heat of gold is
If the specific heat of gold is 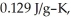 how many joules of heat are absorbed?
how many joules of heat are absorbed?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 41 - 60 of 154
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)