Exam 5: Thermochemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
A 22.9 g sample of iron absorbs 155 J of heat,upon which the temperature of the sample increases from 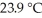 to
to 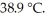 What is the specific heat of iron?
What is the specific heat of iron?
(Multiple Choice)
4.9/5  (37)
(37)
A 5.00-g sample of copper metal at 25.0 °C is heated by the addition of 133 J of energy.The final temperature of the copper is ________ °C.The specific heat capacity of copper is 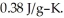
(Multiple Choice)
4.8/5  (29)
(29)
What is the kinetic energy of a 55.2 g object moving at 135 m/s.
(Multiple Choice)
4.8/5  (32)
(32)
Calculate the work (kJ)done during a reaction in which the internal volume expands from 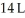 to
to 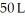 against a vacuum (an outside pressure of
against a vacuum (an outside pressure of 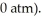
(Multiple Choice)
4.8/5  (33)
(33)
Given the data in the table below,ΔH°rxn for the reaction 4NH3 (g)+ 5O2 (g)→ 4NO (g)+ 6H2O (l)
Is ________ kJ. 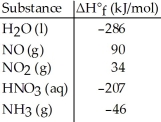
(Multiple Choice)
4.8/5  (32)
(32)
Objects can possess energy as ________. (a)endothermic energy
(b)potential energy
(c)kinetic energy
(Multiple Choice)
4.9/5  (29)
(29)
For the following reactions,the ΔH°rxn is NOT equal to ΔH°f for the product except for ________.
(Multiple Choice)
4.8/5  (33)
(33)
For the following reactions,the ΔH°rxn is NOT equal to ΔH°f for the product except for ________.
(Multiple Choice)
4.8/5  (34)
(34)
Which one of the following conditions would always result in an increase in the internal energy of a system?
(Multiple Choice)
4.8/5  (43)
(43)
A slice of cake contains 29.0 grams of fat,9.0 grams of protein,and 77 grams of carbohydrate.If swimming burns 1000.0 kJ/hour,how many minutes would it take to completely burn off the slice of cake? The respective fuel values for protein,fat,and carbohydrate are 17,38,and 17 kJ/g,respectively.
(Multiple Choice)
5.0/5  (28)
(28)
A 4.50-g sample of liquid water at 25.0 °C is heated by the addition of 133 J of energy.The final temperature of the water is ________ °C.The specific heat capacity of liquid water is 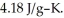
(Multiple Choice)
4.8/5  (30)
(30)
The kinetic energy of a 23.2-g object moving at a speed of 81.9 km/hr is ________ J.
(Multiple Choice)
4.7/5  (31)
(31)
A 10.1 g sample of NaOH is dissolved in 250.0 g of water in a coffee-cup calorimeter.The temperature increases from 23.0 °C to ________°C.Specific heat of liquid water is 4.18 J/g-K and ΔH for the dissolution of sodium hydroxide in water is 44.4 kJ/mol.
(Multiple Choice)
4.8/5  (31)
(31)
Given the following reactions CaCO3 (s)→ CaO (s)+ CO2 (g)ΔH = 178.1 kJ
C (s,graphite)+ O2(g)→ CO2(g)ΔH = -393.5 kJ
The enthalpy of the reaction
CaCO3 (s)→ CaO (s)+ C (s,graphite)+ O2 (g)
Is ________ kJ.
(Multiple Choice)
4.8/5  (37)
(37)
Fuel values of hydrocarbons increase as the ________ increases.
(Multiple Choice)
4.8/5  (38)
(38)
What is the enthalpy change (in kJ)of a chemical reaction that raises the temperature of 250.0 mL of solution having a density of 1.25 g/mL by 3.33 °C? (The specific heat of the solution is 3.74 J/g-K.)
(Multiple Choice)
4.9/5  (33)
(33)
For which one of the following reactions is ΔH°rxn equal to the heat of formation of the product?
(Multiple Choice)
4.9/5  (34)
(34)
The reaction 4Al (s)+ 3O2 (g)→ 2 Al2O3 (s)ΔH° = -3351 kJ
Is ________,and therefore heat is ________ by the reaction.
(Multiple Choice)
4.8/5  (38)
(38)
Given the following reactions 2NO → N2 + O2 ΔH = -180 kJ
2NO + O2 → 2NO2 ΔH = -112 kJ
The enthalpy of the reaction of nitrogen with oxygen to produce nitrogen dioxide
N2 + 2O2 → 2NO2
Is ________ kJ.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 61 - 80 of 154
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)