Exam 5: Thermochemistry
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The value of ΔH° for the reaction below is -336 kJ.Calculate the heat (kJ)released to the surroundings when 23.0 g of HCl is formed. CH4 (g)+ 3Cl2 (g)→ CHCl3 (l)+ 3HCl (g)
(Multiple Choice)
4.8/5  (31)
(31)
The value of ΔH° for the reaction below is -1107 kJ: 2Ba (s)+ O2 (g)→ 2BaO (s)
How many kJ of heat are released when 5.75 g of Ba (s)reacts completely with oxygen to form 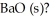
(Multiple Choice)
4.8/5  (36)
(36)
A chemical reaction that releases heat to the surroundings is said to be ________ and has a ________ ΔH at constant pressure.
(Multiple Choice)
4.8/5  (41)
(41)
The kinetic energy of a 7.3 kg steel ball traveling at 18.0 m/s is ________ J.
(Multiple Choice)
4.9/5  (34)
(34)
The value of ΔH° for the reaction below is -1107 kJ: 2Ba (s)+ O2 (g)→ 2BaO (s)
How many kJ of heat are released when 5.75 g of BaO (s)is produced?
(Multiple Choice)
4.8/5  (26)
(26)
The change in the internal energy of a system that releases 2,500 J of heat and that does 7,655 J of work on the surroundings is ________ J.
(Multiple Choice)
4.8/5  (35)
(35)
The ΔHvap of water is 40.7 kJ at 100 °C.How much liquid water in grams can be converted to vapor if 5950 J of heat are absorbed?
(Short Answer)
4.8/5  (37)
(37)
Given the following reactions H2O (l)→ H2O (g)ΔH = 44.01 kJ
2H2 (g)+ O2 (g)→ 2H2O (g)ΔH = -483.64 kJ
The enthalpy for the decomposition of liquid water into gaseous hydrogen and oxygen
2H2O (l)→ 2H2 (g)+ O2 (g)
Is ________ kJ.
(Multiple Choice)
4.8/5  (40)
(40)
The combustion of titanium with oxygen produces titanium dioxide: Ti (s)+ O2(g)→ TiO2 (s)
When 0.610 g of titanium is combusted in a bomb calorimeter,the temperature of the calorimeter increases from 25.00 °C to 50.50 °C.In a separate experiment,the heat capacity of the calorimeter is measured to be 9.84 kJ/K.The heat of reaction for the combustion of a mole of Ti in this calorimeter is ________ kJ/mol.
(Multiple Choice)
4.7/5  (27)
(27)
The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l)→ CO (g)+ 2H2 (g)
How much heat is consumed when 87.1 g of hydrogen gas is formed?
(Multiple Choice)
4.9/5  (40)
(40)
The ________ of a reaction is the enthalpy change when all reactants and products are at 1 atm pressure and a specific temperature.
(Short Answer)
4.8/5  (34)
(34)
What is the molar heat capacity (in J/mol-K)of liquid bromine? The specific heat of liquid bromine is 0.226 J/g-K.
(Multiple Choice)
4.9/5  (31)
(31)
The ΔHvap of water is 40.7 kJ at 100 °C.How much heat energy is required to convert 15.0 grams of liquid water to vapor.
(Short Answer)
4.7/5  (37)
(37)
The value of ΔH° for the reaction below is -72 kJ. ________ kJ of heat are released when 5.5 mol of HBr is formed in this reaction. H2 (g)+ Br2 (g)→ 2HBr (g)
(Multiple Choice)
5.0/5  (37)
(37)
The molar heat capacity of an unknown substance is 92.1 J/mol-K.If the unknown has a molar mass of 118 g/mol,what is the specific heat (J/g-K)of this substance?
(Multiple Choice)
4.8/5  (22)
(22)
The value of ΔH° for the reaction below is -6535 kJ. ________ kJ of heat are released in the combustion of 16.0 g of 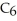
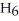 (l)?
2C6H6 (l)+ 15O2 (g)→ 12CO2 (g)+ 6H2O (l)
(l)?
2C6H6 (l)+ 15O2 (g)→ 12CO2 (g)+ 6H2O (l)
(Multiple Choice)
5.0/5  (38)
(38)
________ is defined as the energy used to move an object against a force.
(Short Answer)
4.8/5  (38)
(38)
Showing 21 - 40 of 154
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)