Exam 10: Gases
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Which one of the following is a valid statement of Avogadro's law?
(Multiple Choice)
4.8/5  (40)
(40)
According to kinetic-molecular theory,in which of the following gases will the root-mean-square speed of the molecules be the highest at 200 °C?
(Multiple Choice)
4.9/5  (31)
(31)
How many moles of gas are there in a 36.3 L container at 25.2 °C and 570.3 mm Hg?
(Multiple Choice)
4.8/5  (29)
(29)
Calculate the density of hydrogen gas (in g/L)at 43.0 °C and 700.0 torr.
(Short Answer)
5.0/5  (37)
(37)
A gas vessel is attached to an open-end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below. 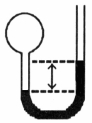 The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
(Multiple Choice)
4.7/5  (37)
(37)
The effusion rate of a gas is proportional to the square root of its molar mass.
(True/False)
4.9/5  (27)
(27)
The amount of gas that occupies 36.52 L at 68.0 °C and 672 mm Hg is ________ mol.
(Multiple Choice)
4.9/5  (36)
(36)
A gas vessel is attached to an open-end manometer containing a nonvolatile liquid of density 0.791 g/mL as shown below. 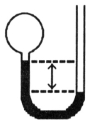 The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg.Given that the density of mercury is 13.6 g/mL,the pressure of the enclosed gas is ________ atm.
(Multiple Choice)
4.9/5  (38)
(38)
The density of nitric oxide (NO)gas at 0.970 atm and 34.9 °C is ________ g/L.
(Multiple Choice)
4.9/5  (28)
(28)
Sodium hydride reacts with excess water to produce aqueous sodium hydroxide and hydrogen gas: NaH (s)+ H2O (l)→ NaOH (aq)+ H2 (g)
A sample of NaH weighing ________ g will produce 982 mL of gas at 28.0 °C and 765 torr,when the hydrogen is collected over water.The vapor pressure of water at this temperature is 28 torr.
(Multiple Choice)
4.9/5  (39)
(39)
Sulfur dioxide (12.4 g)and carbon dioxide (12.4 g)are placed in a 500.0 mL container at 45.0 °C.What is the partial pressure (atm)of sulfur dioxide in the container?
(Multiple Choice)
4.8/5  (34)
(34)
A gas mixture of He and Ar has a total pressure of 4.10 atm.What is the mole fraction of Ar if the partial pressure of Ar is 1.50 atm?
(Multiple Choice)
4.8/5  (31)
(31)
The density of air at STP is 1.285 g/L.Which of the following cannot be used to fill a balloon that will float in air at STP?
(Multiple Choice)
4.8/5  (38)
(38)
If pressure and temperature are kept constant,the reaction of 29 mL of N2 gas with 87 mL of H2 gas will form ________ mL of ammonia.
(Multiple Choice)
4.7/5  (25)
(25)
How many liters of O2 are consumed during the combustion of 60.0 grams of ethane at STP in the open atmosphere?
(Short Answer)
4.8/5  (32)
(32)
A sample of hydrogen gas (3.2 L)at 3.5 atm and 25 °C was combined with 5.2 L of nitrogen gas at 7.3 atm and 25 °C at constant temperature in a 15.0 L flask.Assuming the initial pressure in the flask was 0.00 atm and the temperature upon mixing was 25 °C,what is the total pressure (atm)in the flask?
(Multiple Choice)
4.9/5  (31)
(31)
Showing 121 - 140 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)