Exam 10: Gases
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
SO2 (5.00 g)and CO2 (5.00 g)were placed in a 750.0 mL container at 50.0 °C.The partial pressure of CO2 in the container was ________ atm.
(Multiple Choice)
4.8/5  (45)
(45)
Arrange the following gases in order of increasing average molecular speed at 25 °C. He,O2,CO2,N2
(Multiple Choice)
4.8/5  (32)
(32)
If pressure and temperature are kept constant,the reaction of 38 mL of Cl2 gas with 22 mL of CH4 gas via the equation: Cl2 (g)+ CH4 (g)→ HCl (g)+ CH3Cl (g)
Will produce a total of ________ mL of products.
(Multiple Choice)
4.8/5  (33)
(33)
What volume (mL)of sulfur dioxide can be produced by the complete reaction of 3.82 g of calcium sulfite with excess HCl (aq),when the final SO2 pressure is 827 torr at 44.0 °C?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following equations shows an incorrect relationship between pressures given in terms of different units?
(Multiple Choice)
4.9/5  (32)
(32)
Which one of the following gases would deviate the least from ideal gas behavior?
(Multiple Choice)
4.9/5  (34)
(34)
A gas originally at 27 °C and 1.00 atm pressure in a 3.3 L flask is cooled at constant pressure until the temperature is 11 °C.The new volume of the gas is ________ L.
(Multiple Choice)
4.9/5  (35)
(35)
If 50.75 g of a gas occupies 10.0 L at STP,129.3 g of the gas will occupy ________ L at STP.
(Multiple Choice)
4.9/5  (33)
(33)
A balloon originally had a volume of 4.39 L at 44 °C and a pressure of 729 torr.The balloon must be cooled to ________ °C to reduce its volume to 3.99 L (at constant pressure).
(Multiple Choice)
4.7/5  (34)
(34)
The average kinetic energy of the particles of a gas is ________ proportional to ________.
(Multiple Choice)
4.9/5  (28)
(28)
Two deviations of real gases from ideal gases which are treated in the van der Waals equation are finite molecular volume and non-zero molecular attractions.
(True/False)
4.8/5  (30)
(30)
A fixed amount of gas at 25.0 °C occupies a volume of 10.0 L when the pressure is 751 torr.Use Boyle's law to calculate the pressure (torr)when the volume is reduced to 7.25 L at a constant temperature of 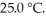
(Multiple Choice)
4.7/5  (33)
(33)
A gas mixture of N2 and H2 has a total pressure of 9.40 atm and contains 11.3 mol of gas.If the partial pressure of N2 is 4.89 atm,how many moles of H2 are in the mixture?
(Multiple Choice)
4.8/5  (31)
(31)
A sample of CO2 gas (3.0 mol)effused through a pinhole in 18.0 s.It will take ________ s for the same amount of H2 to effuse under the same conditions.
(Multiple Choice)
4.9/5  (40)
(40)
A flask contains a mixture of N2 and H2 at a total pressure of 5.20 atm.If there are 1.00 mol of H2 and 8.00 mol of N2 in the flask,what is the partial pressure (atm)of N2?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 81 - 100 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)