Exam 10: Gases
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The pressure of a sample of CH4 gas (6.022 g)in a 30.0 L vessel at 402 K is ________ atm.
(Multiple Choice)
4.9/5  (35)
(35)
The volume occupied by 1.5 mol of gas at 35 °C and 2.0 atm pressure is ________ L.
(Multiple Choice)
4.8/5  (43)
(43)
If pressure and temperature are kept constant,the reaction of 18 mL of N2 gas with 54 mL of H2 gas will form ________ mL of ammonia.
(Multiple Choice)
4.8/5  (35)
(35)
At a temperature of ________ °C,0.444 mol of CO gas occupies 11.8 L at 839 torr.
(Multiple Choice)
4.8/5  (35)
(35)
What is the total pressure (atm)in a 10.0 L vessel that contains 2.34 mol of carbon dioxide,1.73 mol of sulfur dioxide,and 4.50 mol of argon at standard temperature?
(Multiple Choice)
4.7/5  (40)
(40)
Since air is a mixture,it does not have a "molar mass." However,for calculation purposes,it is possible to speak of its "effective molar mass." (An effective molar mass is a weighted average of the molar masses of a mixture's components.)If air at STP has a density of 1.285 g/L,its effective molar mass is ________ g/mol.
(Multiple Choice)
4.8/5  (33)
(33)
A sample of He gas (2.0 mmol)effused through a pinhole in 53 s.The same amount of an unknown gas,under the same conditions,effused through the pinhole in 248 s.The molecular mass of the unknown gas is ________ g/mol.
(Multiple Choice)
4.9/5  (34)
(34)
The molecular weight of a gas is ________ g/mol if 3.5 g of the gas occupies 2.1 L at STP.
(Multiple Choice)
4.7/5  (38)
(38)
5.25 g of zinc metal reacts with excess sulfuric acid to produce hydrogen gas.What volume (L)of hydrogen is generated at STP?
(Multiple Choice)
4.8/5  (36)
(36)
A 5.50 L vessel contains 0.348 mol of methane,0.311 mol of propane,and 0.445 mol of neon at a total pressure of 882 mm Hg.What is the partial pressure (in mm Hg)of methane?
(Short Answer)
4.8/5  (35)
(35)
A pressure vessel contains CO2 (PCO2 = 3.78 atm)and O2 (PO2 = 6 atm)gases at a total pressure of 9.78 atm.What is the mole-fraction of CO2 and O2 gases,respectively?
(Multiple Choice)
4.8/5  (27)
(27)
A fixed amount of gas at 25.0 °C occupies a volume of 8.66 L when the pressure is 629 torr.Use Charles's law to calculate the volume (L)the gas will occupy when the temperature is increased to 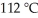 while maintaining the pressure at 629 torr.
while maintaining the pressure at 629 torr.
(Multiple Choice)
4.8/5  (35)
(35)
A 255 mL round-bottom flask is weighed and found to have a mass of 114.85 g.A few milliliters of an easily vaporized liquid are added to the flask and the flask is immersed in a boiling water bath.All of the liquid vaporizes at the boiling temperature of water,filling the flask with vapor.When all of the liquid has vaporized,the flask is removed from the bath,cooled,dried,and reweighed.The new mass of the flask and the condensed vapor is 115.23 g.Which of the following compounds could the liquid be? (Assume the ambient pressure is 1 atm.)
(Multiple Choice)
4.8/5  (43)
(43)
A sample of Ne gas (2.5 L)at 4.5 atm and 25 °C was combined with 2.2 L of Ar gas at 6.3 atm and 25 °C at constant temperature in a 8.0 L flask.Assuming the initial pressure in the flask was 0.00 atm and the temperature upon mixing was 25 °C,what is the total pressure (atm)in the flask?
(Multiple Choice)
4.8/5  (32)
(32)
A pressure of 0.500 atm is the same as a pressure of ________ of mm Hg.
(Multiple Choice)
4.8/5  (38)
(38)
A 0.325 L flask filled with gas at 0.851 atm and 19 °C contains ________ mol of gas.
(Multiple Choice)
4.7/5  (37)
(37)
Showing 41 - 60 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)