Exam 10: Gases
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
What volume (L)of fluorine gas is required to react with 2.31 g of calcium bromide to form calcium fluoride and bromine gas at 8.19 atm and 35.0 °C?
(Multiple Choice)
4.9/5  (37)
(37)
A sample of an unknown volatile liquid was injected into a Dumas flask 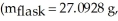
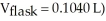 and heated until no visible traces of the liquid could be found.The flask and its contents were then rapidly cooled and reweighed
and heated until no visible traces of the liquid could be found.The flask and its contents were then rapidly cooled and reweighed 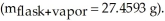 The atmospheric pressure and temperature during the experiment were 0.976 atm and 18.0 °C,respectively.The unknown volatile liquid was ________.
The atmospheric pressure and temperature during the experiment were 0.976 atm and 18.0 °C,respectively.The unknown volatile liquid was ________.
(Multiple Choice)
4.8/5  (34)
(34)
A sample of an ideal gas (3.00 L)in a closed container at 25.0 °C and 76.0 torr is heated to 270 °C.The pressure of the gas at this temperature is ________ torr.
(Multiple Choice)
4.9/5  (38)
(38)
A gas mixture of Xe,Ne,and Ar has a total pressure of 12.20 atm.What is the mole fraction of Xe if the partial pressures of Ne and Ar are 2.10 and 4.50 atm,respectively?
(Multiple Choice)
4.8/5  (21)
(21)
Of the following,________ is a correct statement of Boyle's law.
(Multiple Choice)
4.8/5  (34)
(34)
How many moles of an unknown gas are in a 325 mL container at a pressure of 695 torr and 19 °C?
(Multiple Choice)
4.8/5  (31)
(31)
What is the density of carbon dioxide gas (g/L)at 1106.2 mm Hg and 56.3 °C?
(Multiple Choice)
4.8/5  (40)
(40)
What is the molecular weight (g/mol)of an unknown gas that has a density of 3.59 g/L at STP?
(Multiple Choice)
4.8/5  (38)
(38)
The pressure exerted by a column of liquid is equal to the product of the height of the column times the gravitational constant times the density of the liquid, 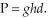 How high a column of methanol
How high a column of methanol 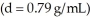 would be supported by a pressure that supports a 713 mm column of mercury
would be supported by a pressure that supports a 713 mm column of mercury 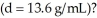
(Multiple Choice)
4.8/5  (32)
(32)
The density of krypton gas at 0.866 atm and 44.4 °C is ________ g/L.
(Multiple Choice)
4.9/5  (41)
(41)
A sample of a gas (1.50 mol)is contained in a 15.0 L cylinder.The temperature is increased from 100 °C to 150 °C.The ratio of final pressure to initial pressure [ ![A sample of a gas (1.50 mol)is contained in a 15.0 L cylinder.The temperature is increased from 100 °C to 150 °C.The ratio of final pressure to initial pressure [ ] is ________.](https://storage.examlex.com/TB1194/11ea7e7c_612c_7806_9a0a_a3fc5d19f6f4_TB1194_11.jpg) ] is ________.
] is ________.
(Multiple Choice)
4.8/5  (25)
(25)
The root-mean-square speed of CO at 113 °C is ________ m/s.
(Multiple Choice)
4.9/5  (28)
(28)
10.0 grams of argon and 20.0 grams of neon are placed in a 1216.1 ml container at 25.1 °C.The partial pressure of neon is ________ atm.
(Multiple Choice)
4.9/5  (29)
(29)
CO (5.00 g)and CO2 (5.00 g)were placed in a 750.0 mL container at 50.0 °C.The partial pressure of CO in the container was ________ atm.
(Multiple Choice)
4.8/5  (34)
(34)
The deviation from ideal behavior of a gas is most evident at ________ and/or low temperature.
(Short Answer)
4.9/5  (32)
(32)
A sample of H2 gas (12.28 g)occupies 100.0 L at 400.0 K and 2.00 atm.A sample weighing 9.49 g occupies ________ L at 353 K and 2.00 atm.
(Multiple Choice)
4.8/5  (31)
(31)
A sample of a gas originally at 29 °C and 1.25 atm pressure in a 3.0 L container is allowed to contract until the volume is 2.2 L and the temperature is 11 °C.The final pressure of the gas is ________ atm.
(Multiple Choice)
4.9/5  (29)
(29)
Of the following gases,________ will have the greatest rate of effusion at a given temperature.
(Multiple Choice)
4.8/5  (36)
(36)
A gas is considered "ideal" if one mole of it in a one-liter container exerts a pressure of exactly 1 atm at room temperature.
(True/False)
4.7/5  (26)
(26)
Showing 101 - 120 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)