Exam 10: Gases
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Using the van der Waals equation,the pressure in a 22.4 L vessel containing 1.50 mol of chlorine gas at 0.00 °C is ________ atm. (a = 6.49 L2-atm/mol2,b = 0.0562 L/mol)
(Multiple Choice)
4.8/5  (34)
(34)
The volume of a sample of gas (2.49 g)was 752 mL at 1.98 atm and 62 °C.The gas is ________.
(Multiple Choice)
4.8/5  (38)
(38)
A gas at a pressure of 10.0 Pa exerts a force of ________ N on an area of 5.5 m2.
(Multiple Choice)
4.8/5  (33)
(33)
Ammonium nitrite undergoes thermal decomposition to produce only gases: NH4NO2 (s)→ N2 (g)+ 2H2O (g)
What volume (L)of gas is produced by the decomposition of 35.0 g of NH4NO2(s)at 525 °C and 1.5 atm?
(Multiple Choice)
4.9/5  (33)
(33)
The volume of HCl gas required to react with excess magnesium metal to produce 6.82 L of hydrogen gas at 2.19 atm and 35.0 °C is ________ L.
(Multiple Choice)
4.9/5  (28)
(28)
A sample of oxygen gas was found to effuse at a rate equal to two times that of an unknown gas.The molecular weight of the unknown gas is ________ g/mol.
(Multiple Choice)
4.8/5  (37)
(37)
Which noble gas is expected to show the largest deviations from the ideal gas behavior?
(Multiple Choice)
4.8/5  (30)
(30)
The volume of 0.15 mol of an ideal gas at 365 torr and 97 °C is ________ L.
(Multiple Choice)
4.9/5  (34)
(34)
Standard temperature and pressure (STP),in the context of gases,refers to ________.
(Multiple Choice)
4.9/5  (28)
(28)
What volume (L)of NH3 gas at STP is produced by the complete reaction of 7.5 g of H2O according to the following reaction?
Mg3N2 (s)+ 6H2O (l)→ 3Mg(OH)2 (aq)+ 2NH3 (g)
(Multiple Choice)
4.9/5  (38)
(38)
How much CO2 (L)is produced when 2.10 kg of sodium bicarbonate reacts with excess hydrochloric acid at 25.0 °C and 1.23 atm?
(Multiple Choice)
4.8/5  (37)
(37)
Calcium hydride ( 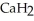 )reacts with water to form hydrogen gas:
)reacts with water to form hydrogen gas: 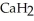 (s)+
(s)+ 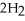 O (l)→
O (l)→ 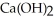 (aq)+
(aq)+ 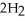 (g) How many grams of
(g) How many grams of 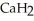 are needed to generate 48.0 L of
are needed to generate 48.0 L of 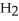 gas at a pressure of 0.995 atm and a temperature of 32 °C?
gas at a pressure of 0.995 atm and a temperature of 32 °C?
(Multiple Choice)
4.9/5  (28)
(28)
A real gas will behave most like an ideal gas under conditions of ________.
(Multiple Choice)
4.9/5  (38)
(38)
A gas in a 57.1 L pressure container at 24.9 °C and 889.8 mm Hg contains ________ moles.
(Multiple Choice)
4.7/5  (43)
(43)
Which one of the following gases would have the highest average molecular speed at 25 °C?
(Multiple Choice)
4.8/5  (36)
(36)
1.92 g of zinc metal reacts with excess hydrochloric acid to produce hydrogen gas.What volume (L)of hydrogen is generated at STP?
(Multiple Choice)
4.8/5  (33)
(33)
Kinetic-molecular theory assumes that attractive and repulsive forces between gas particles are stronger than those between gas particles and container walls.
(True/False)
4.8/5  (36)
(36)
34.9 grams of hydrogen gas and 17.7 grams of methane gas are combined in a reaction vessel with a total pressure at 2.92 atm.What is the partial pressure (atm)of hydrogen gas?
(Multiple Choice)
4.9/5  (33)
(33)
Showing 141 - 160 of 175
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)