Exam 13: Properties of Solutions
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
After swimming in the ocean for several hours,swimmers noticed that their fingers appeared to be very wrinkled.This is an indication that seawater is supertonic relative to the fluid in cells.
(True/False)
4.7/5  (38)
(38)
Pressure has an appreciable effect on the solubility of ________ in liquids.
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the freezing point of a solution containing 20 grams of KCl and 2200.0 grams of water.The molal-freezing-point-depression constant ( 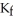 )for water is
)for water is 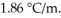 (Assume 100% ionization of KCl.)
(Assume 100% ionization of KCl.)
(Multiple Choice)
4.7/5  (35)
(35)
What is the molality of a 36.1% (by mass)aqueous solution of phosphoric acid?
(Multiple Choice)
4.9/5  (36)
(36)
The molarity of urea (MW = 60.0 g/mol)in a solution prepared by dissolving 11 g of urea in 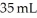 of H2O is ________ M.Assume the volume of the solution does not change when dissolving the urea.
of H2O is ________ M.Assume the volume of the solution does not change when dissolving the urea.
(Multiple Choice)
4.8/5  (41)
(41)
A 0.200 m solution of which one of the following solutes will have the lowest vapor pressure?
(Multiple Choice)
4.9/5  (34)
(34)
An aqueous solution of a soluble compound (a nonelectrolyte)is prepared by dissolving 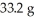 of the compound in sufficient water to form
of the compound in sufficient water to form 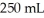 of solution.The solution has an osmotic pressure of 1.2 atm at
of solution.The solution has an osmotic pressure of 1.2 atm at 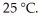 What is the molar mass (g/mole)of the compound?
What is the molar mass (g/mole)of the compound?
(Multiple Choice)
4.9/5  (35)
(35)
Emulsifying agents typically have a hydrophobic end and a hydrophilic end.
(True/False)
4.9/5  (40)
(40)
The concentration of lead nitrate (Pb(N 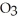
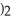 )in a 0.926 M solution is ________ molal.The density of the solution is 1.202 g/mL.
)in a 0.926 M solution is ________ molal.The density of the solution is 1.202 g/mL.
(Multiple Choice)
4.8/5  (35)
(35)
What is the molality of a 10.0% (by mass)aqueous solution of hydrochloric acid?
(Multiple Choice)
4.8/5  (30)
(30)
A solution is prepared by dissolving 2.00 g of glycerin ( 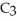
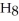
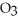 )in 201 g of ethanol
)in 201 g of ethanol 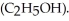 The freezing point of the solution is ________°C.The freezing point of pure ethanol is
The freezing point of the solution is ________°C.The freezing point of pure ethanol is 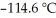 at 1 atm.The molal-freezing-point-depression constant (
at 1 atm.The molal-freezing-point-depression constant ( 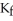 )for ethanol is
)for ethanol is 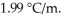 The molar masses of glycerin and of ethanol are 92.1 g/mol and 46.1 g/mol,respectively.
The molar masses of glycerin and of ethanol are 92.1 g/mol and 46.1 g/mol,respectively.
(Multiple Choice)
4.7/5  (32)
(32)
Which produces the greatest number of ions when one mole dissolves in water?
(Multiple Choice)
4.7/5  (38)
(38)
What is the molality of a 24.4% (by mass)aqueous solution of phosphoric acid?
(Multiple Choice)
4.8/5  (39)
(39)
The concentration of CO2 in a soft drink bottled with a partial pressure of CO2 of 6.5 atm over the liquid at 29 °C is 2.2 × 10-1 M.The Henry's law constant for CO2 at this temperature is ________.
(Multiple Choice)
4.8/5  (28)
(28)
What is the molality of LiCl in solution that is 9.0% by mass LiCl and has a density of 1.00 g/mL?
(Multiple Choice)
4.7/5  (41)
(41)
What is the molarity of phosphoric acid in a 31.5% (by mass)aqueous solution?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following liquids will have the lowest freezing point?
(Multiple Choice)
4.9/5  (37)
(37)
The value of the boiling-point-elevation constant (Kb)depends on the identity of the solvent.
(True/False)
4.9/5  (35)
(35)
The process of a substance sticking to the surface of another is called ________.
(Multiple Choice)
4.9/5  (39)
(39)
Showing 21 - 40 of 160
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)