Exam 13: Properties of Solutions
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The magnitudes of Kf and of Kb depend on the identity of the ________.
(Multiple Choice)
5.0/5  (38)
(38)
A solution contains 15 ppm of benzene.The density of the solution is 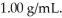 This means that ________.
This means that ________.
(Multiple Choice)
4.8/5  (32)
(32)
On a clear day at sea level,with a temperature of 25 °C,the partial pressure of 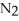 in air is 0.78 atm and the concentration of nitrogen in water is
in air is 0.78 atm and the concentration of nitrogen in water is 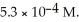 When the partial pressure of
When the partial pressure of 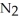 is ________ atm,the concentration in water is 2.0
is ________ atm,the concentration in water is 2.0 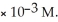
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following substances is least likely to dissolve in water?
(Multiple Choice)
4.9/5  (24)
(24)
An acetic acid aqueous solution contains 22% by mass acetic acid? Which of the following statements is correct?
(Multiple Choice)
4.9/5  (31)
(31)
A solution is prepared by dissolving 27.7 g of Ca 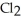 in 375 g of water.The density of the resulting solution is 1.05 g/mL.The concentration of Ca
in 375 g of water.The density of the resulting solution is 1.05 g/mL.The concentration of Ca 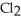 is ________% by mass.
is ________% by mass.
(Multiple Choice)
4.8/5  (28)
(28)
If the partial pressure of oxygen in the air a diver breathes is too great,________.
(Multiple Choice)
4.8/5  (46)
(46)
A mixture containing 41.0 g of an unknown nonelectrolyte and 160.0 g of water has a freezing point of -1.34 °C.Given 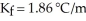 for water,what is the molecular weight (g/mol)of the unknown solute?
for water,what is the molecular weight (g/mol)of the unknown solute?
(Multiple Choice)
4.9/5  (32)
(32)
Colligative properties of solutions include all of the following except ________.
(Multiple Choice)
4.8/5  (37)
(37)
A solution is prepared by dissolving 23.7 g of CaCl2 in 375 g of water.The density of the resulting solution is 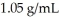 .The concentration of Cl- in this solution is ________ M.
.The concentration of Cl- in this solution is ________ M.
(Multiple Choice)
4.8/5  (26)
(26)
A 30.0 g sample of potassium nitrate is dissolved in 100 g of water at 60 °C.The solution is cooled to 20.0 °C and a small amount of precipitate is observed.This solution is ________.
(Multiple Choice)
4.9/5  (36)
(36)
The mole fraction of He in a gaseous solution prepared from 1.0 g of He,6.5 g of Ar,and 10.0 g of Ne is ________.
(Multiple Choice)
4.7/5  (32)
(32)
What is the mole fraction of N 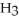 in a solution prepared by dissolving 16.0 g of N
in a solution prepared by dissolving 16.0 g of N 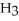 in 250.0 g of water? The density of the resulting solution is 0.974 g/mL.
in 250.0 g of water? The density of the resulting solution is 0.974 g/mL.
(Multiple Choice)
4.7/5  (34)
(34)
A solution contains 11% by mass of sodium chloride.This means that ________.
(Multiple Choice)
4.9/5  (31)
(31)
A solution is prepared by dissolving 0.60 g of nicotine (a nonelectrolyte)in water to make 12 mL of solution.The osmotic pressure of the solution is 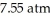 at 25 °C.The molecular weight of nicotine is
at 25 °C.The molecular weight of nicotine is 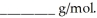
(Multiple Choice)
4.8/5  (36)
(36)
Calculate the molarity of a 17.5% (by mass)aqueous solution of nitric acid.
(Multiple Choice)
4.9/5  (33)
(33)
A 17.2 g sample of potassium chlorate is dissolved in 250 g of water at 65 °C.The solution is cooled to 30.0 °C and no precipitate is observed.This solution is ________.
(Multiple Choice)
4.8/5  (32)
(32)
The ratio of the actual value of a colligative property to the value calculated,assuming the substance to be a nonelectrolyte,is referred to as ________.
(Multiple Choice)
4.9/5  (33)
(33)
Showing 101 - 120 of 160
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)