Exam 18: Chemistry of the Environment
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Which of the following is not one of the 12 principles associated with green chemistry?
(Multiple Choice)
5.0/5  (35)
(35)
What compound in limestone and marble is attacked by acid rain?
(Multiple Choice)
4.7/5  (42)
(42)
Why are chlorofluorocarbons so damaging to the ozone layer when they are such stable molecules?
(Multiple Choice)
4.7/5  (35)
(35)
Which gaseous sulfur compound combines with water to form the principal acidic constituent of acid rain?
(Multiple Choice)
4.7/5  (40)
(40)
Which choice is not one of the Ionic Constituents of seawater typically present in concentrations greater than 1 ppm?
(Multiple Choice)
4.9/5  (36)
(36)
Which choice is one of the suspected carcinogens by-products from water chlorination called THMs?
(Multiple Choice)
4.9/5  (32)
(32)
In the equation below,what is the meaning of the asterisk?
O + O2 → O3*
(Essay)
4.9/5  (31)
(31)
Sulfur dioxide is not released into the atmosphere in any significant amount by ________.
(Multiple Choice)
5.0/5  (28)
(28)
The majority of ozone that protects against the high energy radiation of the sun is found in the ________.
(Multiple Choice)
4.8/5  (35)
(35)
What is the wavelength of a photon with just enough energy to break a 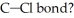 (The bond energy of the
(The bond energy of the 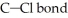 is
is 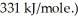
(Multiple Choice)
4.8/5  (34)
(34)
The stratosphere contains approximately ________ of the earth's ozone.
(Short Answer)
4.7/5  (30)
(30)
The brown color of photochemical smog over a city is mainly due to ________.
(Multiple Choice)
4.8/5  (35)
(35)
Carbon dioxide contributes to atmospheric warming by ________.
(Multiple Choice)
4.8/5  (28)
(28)
A single individual typically uses the greatest quantity of water for ________.
(Multiple Choice)
4.9/5  (36)
(36)
Ozone is a necessary,protective component of the ________,but is considered a pollutant in the ________.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 81 - 100 of 126
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)