Exam 18: Chemistry of the Environment
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Municipal water treatment consists of five steps ending with chlorination.
(True/False)
4.8/5  (31)
(31)
Nitric oxide arises from auto emissions and reacts with oxygen to produce ________ which reacts with sunlight to produce ________ atoms which react with oxygen to produce ________.
(Short Answer)
4.7/5  (36)
(36)
Which of the following is (are)not a typical source of nitric oxide?
(Multiple Choice)
4.8/5  (35)
(35)
What is the concentration (ppm)of water vapor in a sample of air that has a partial pressure of water of 1.20 torr and a total pressure of air of 695 torr?
(Multiple Choice)
4.9/5  (32)
(32)
The total pressure of a component in a gas mixture is the product of its mole fraction and the partial mixture pressure.
(True/False)
4.7/5  (33)
(33)
Of the following,only ________ does not result in the formation of acid rain.
(Multiple Choice)
4.7/5  (29)
(29)
Components of Air Mole Fraction Nitrogen 0.781
Oxygen 0.209
Argon 0.010
What is the partial pressure or nitrogen (in torr)in the atmosphere when the atmospheric pressure is 760.0 torr?
(Multiple Choice)
4.9/5  (32)
(32)
The mole fraction of nitrogen in dry air near sea level is 0.78084,where the molar mass of nitrogen is 28.013.The partial pressure of nitrogen when the total atmospheric pressure (dry air)is 97.5 kPa is 
(Multiple Choice)
4.9/5  (41)
(41)
The concentration of 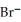 in a sample of seawater is 5.3 ×
in a sample of seawater is 5.3 × 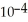 M.What is the concentration (ppm)of
M.What is the concentration (ppm)of 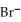 ?
?
(Multiple Choice)
4.8/5  (33)
(33)
The lime-soda process is ________ being added to water to cause precipitation of magnesium as Mg(OH)2 and is used for large-scale water-softening operations.
(Multiple Choice)
4.8/5  (40)
(40)
In the troposphere,temperature ________ with increasing altitude,while in the stratosphere,temperature ________ with increasing altitude.
(Multiple Choice)
4.9/5  (30)
(30)
What is/are the product(s)of photodissociation of molecular oxygen?
(Multiple Choice)
4.8/5  (37)
(37)
In the equation below,M is most likely ________. O + O2 + M → O3 + M*
(Multiple Choice)
4.8/5  (31)
(31)
Ozone depletion from chlorofluorocarbons is chiefly due to the production of free chlorine.
(True/False)
4.8/5  (32)
(32)
What are the primary chemical pollutants that create acid rain?
(Short Answer)
4.8/5  (43)
(43)
What is the total pressure (torr)inside a container that contains a gas at 3.18 × 103 ppm where its partial pressure is 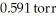 ?
?
(Multiple Choice)
4.8/5  (40)
(40)
The mole fraction of nitrous oxide in dry air near sea level is 0.00000050.The concentration of nitrous oxide is ________ molecules per liter,assuming an atmospheric pressure of 739 torr and a temperature of 29.5 °C.
(Multiple Choice)
4.8/5  (35)
(35)
Show how a molecule of C2F2Cl2 can destroy two molecules of ozone,O3.
(Essay)
4.7/5  (28)
(28)
The cumulative result of carbon dioxide,methane,and ozone in the troposphere on atmospheric temperatures is referred to as ________.
(Multiple Choice)
4.9/5  (27)
(27)
Showing 41 - 60 of 126
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)