Exam 18: Chemistry of the Environment
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
Which one of the following is produced by anaerobic bacteria after decomposing biodegradable nitrogen-containing waste?
(Multiple Choice)
4.9/5  (21)
(21)
The C-Cl and C-F bond dissociation energies in CF3Cl are 339 kJ/mol and 482 kJ/mol,respectively.The maximum wavelengths of electromagnetic radiation required to rupture these bonds are ________ and ________,respectively.
(Multiple Choice)
4.7/5  (29)
(29)
The dissociation energy of N2 is 941 kJ/mol.The maximum wavelength of light that has enough energy per photon to dissociate the N2 molecule is ________ nm.
(Multiple Choice)
4.8/5  (32)
(32)
Of the noble gases,________ is present in highest concentration in dry air at sea level.
(Multiple Choice)
4.9/5  (38)
(38)
The acidic water of a lake (V = 4 × 108 L)must be neutralized with lime,CaO.It will require a minimum of ________ grams of lime to adjust the pH from 5.6 to 6.5.
(Multiple Choice)
5.0/5  (29)
(29)
The concentration of ozone in a sample of air that has a partial pressure of O3 of 0.53 torr and a total pressure of air of 695 torr is ________ ppm.
(Multiple Choice)
4.9/5  (36)
(36)
Of the compounds below,the one that requires the shortest wavelength for photoionization is ________.
(Multiple Choice)
4.8/5  (23)
(23)
The ionization energy of NO is 890 kJ/mol: NO + hv → NO+ + 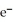 The maximum wavelength of light capable of causing the ionization of NO is ________ nm.
The maximum wavelength of light capable of causing the ionization of NO is ________ nm.
(Multiple Choice)
5.0/5  (28)
(28)
Incomplete combustion of carbon-containing materials occurs when ________.
(Multiple Choice)
4.7/5  (38)
(38)
In the presence of oxygen,the nitrogen present in biodegradable material ends up mainly as ________.
(Multiple Choice)
4.8/5  (26)
(26)
The concept that radiowave propagation was affected by the atmosphere of the earth was discovered by ________.
(Short Answer)
4.9/5  (37)
(37)
In the purification of drinking water,the filtered water is made basic by adding ________,then particles and bacteria are captured by adding Al2(SO4)3.
(Short Answer)
4.8/5  (42)
(42)
What is the concentration (ppm)of helium in the atmosphere if the mole fraction of helium in dry air near sea level is 0.00000524?
(Multiple Choice)
4.8/5  (39)
(39)
How many liters of water does the average adult in the U.S.require?
(Short Answer)
5.0/5  (35)
(35)
Compounds found in fossil fuels that contain ________ are primarily responsible for acid rain.
(Multiple Choice)
4.9/5  (20)
(20)
Showing 21 - 40 of 126
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)