Exam 18: Chemistry of the Environment
Exam 1: Introduction: Matter, energy, and Measurement163 Questions
Exam 2: Atoms, molecules, and Ions249 Questions
Exam 3: Chemical Reactions and Reaction Stoichiometry178 Questions
Exam 4: Reactions in Aqueous Solution178 Questions
Exam 5: Thermochemistry154 Questions
Exam 6: Electronic Structure of Atoms186 Questions
Exam 7: Periodic Properties of the Elements176 Questions
Exam 8: Basic Concepts of Chemical Bonding146 Questions
Exam 9: Molecular Geometry and Bonding Theories183 Questions
Exam 10: Gases175 Questions
Exam 11: Liquids and Intermolecular Forces124 Questions
Exam 12: Solids and Modern Materials84 Questions
Exam 13: Properties of Solutions160 Questions
Exam 14: Chemical Kinetics134 Questions
Exam 15: Chemical Equilibrium97 Questions
Exam 16: Acid-Base Equilibria139 Questions
Exam 17: Additional Aspects of Aqueous Equilibria116 Questions
Exam 18: Chemistry of the Environment126 Questions
Exam 19: Chemical Thermodynamics125 Questions
Exam 20: Electrochemistry113 Questions
Exam 21: Nuclear Chemistry178 Questions
Exam 22: Chemistry of the Nonmetals194 Questions
Exam 23: Transition Metals and Coordination Chemistry165 Questions
Exam 24: The Chemistry of Life: Organic and Biological Chemistry131 Questions
Select questions type
The concentration of which greenhouse gas has increased steadily over the last few decades?
(Multiple Choice)
4.9/5  (35)
(35)
The greenhouse effect of a methane molecule far exceeds the effect of a carbon dioxide molecule.
(True/False)
4.7/5  (31)
(31)
What is the total pressure (torr)inside a container that contains a gas at 8.57 × 103 ppm where its partial pressure is 1.87 torr?
(Multiple Choice)
4.9/5  (37)
(37)
Organic material that bacteria are able to oxidize is known as ________.
(Short Answer)
4.7/5  (38)
(38)
________ and ________ are major contributors to the depletion of the ozone layer.
(Short Answer)
4.9/5  (39)
(39)
Which of the following is arranged correctly in order of increasing distance from Earth's surface?
(Multiple Choice)
4.7/5  (42)
(42)
Of the reactions involved in the photodecomposition of ozone (shown below),which are photochemical?
1.O2 (g)+ hν → O (g)+ O (g)
2.O (g)+ O2 (g)+ M (g)→ O3 (g)+ M* (g)
3.O3 (g)+ hν → O2 (g)+ O (g)
4.O (g)+ O (g)+ M (g)→ O2 (g)+ M* (g)
(Multiple Choice)
4.8/5  (29)
(29)
The area of the Earth's atmosphere 50 - 85 km above the surface is called the ________.
(Multiple Choice)
5.0/5  (28)
(28)
Sulfur compounds in the atmosphere are equally derived from natural sources and from human activity.
(True/False)
4.8/5  (42)
(42)
Which one of the following is a source of carbon dioxide in the troposphere?
(Multiple Choice)
4.8/5  (36)
(36)
Clean rainwater is ________ mainly due to the presence of carbon dioxide.
(Short Answer)
4.9/5  (33)
(33)
Describe the process of reverse osmosis that is used to desalinate seawater.
(Essay)
4.9/5  (39)
(39)
Ozone is more efficient at killing bacteria in water,yet chlorine is used more commonly for that purpose in municipal water treatment.Why?
(Essay)
4.8/5  (34)
(34)
Water containing high concentrations of ________ cations is called hard water.
(Multiple Choice)
4.8/5  (35)
(35)
Which process in the Global Water Cycle involves the phase transition 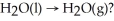
(Multiple Choice)
4.9/5  (29)
(29)
Showing 101 - 120 of 126
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)