Exam 18: Thermal Properties of Matter
Exam 1: Units,physical Quantities,and Vectors107 Questions
Exam 2: Motion Along a Straight Line59 Questions
Exam 3: Motion in Two or Three Dimensions50 Questions
Exam 4: Newtons Laws of Motion44 Questions
Exam 5: Applying Newtons Laws95 Questions
Exam 6: Work and Kinetic Energy54 Questions
Exam 7: Potential Energy and Energy Conservation55 Questions
Exam 8: Momentum,impulse,and Collisions50 Questions
Exam 9: Rotation of Rigid Bodies26 Questions
Exam 10: Dynamics of Rotational Motion49 Questions
Exam 11: Equilibrium and Elasticity50 Questions
Exam 12: Fluid Mechanics50 Questions
Exam 13: Gravitation50 Questions
Exam 14: Periodic Motion50 Questions
Exam 15: Mechanical Waves44 Questions
Exam 16: Sound and Hearing65 Questions
Exam 17: Temperature and Heat63 Questions
Exam 18: Thermal Properties of Matter58 Questions
Exam 19: The First Law of Thermodynamics52 Questions
Exam 20: The Second Law of Thermodynamics50 Questions
Exam 21: Electric Charge and Electric Field60 Questions
Exam 22: Gausss Law41 Questions
Exam 23: Electric Potential55 Questions
Exam 24: Capacitance and Dielectrics52 Questions
Exam 25: Current,resistance,and Electromotive Force55 Questions
Exam 26: Direct-Current Circuits53 Questions
Exam 27: Magnetic Field and Magnetic Forces42 Questions
Exam 28: Sources of Magnetic Field52 Questions
Exam 29: Electromagnetic Induction39 Questions
Exam 30: Inductance27 Questions
Exam 31: Alternating Current50 Questions
Exam 32: Electromagnetic Waves47 Questions
Exam 33: The Nature and Propagation of Light28 Questions
Exam 34: Geometric Optics81 Questions
Exam 35: Interference33 Questions
Exam 36: Diffraction49 Questions
Exam 37: Relativity51 Questions
Exam 38: Photons: Light Waves Behaving As Particles38 Questions
Exam 39: Particles Behaving As Waves52 Questions
Exam 40: Quantum Mechanics43 Questions
Exam 41: Atomic Structure53 Questions
Exam 42: Molecules and Condensed Matter31 Questions
Exam 43: Nuclear Physics90 Questions
Exam 44: Particle Physics and Cosmology54 Questions
Select questions type
An ideal gas is kept in a rigid container that expands negligibly when heated.The gas starts at a temperature of 20.0°C,and heat is added to increase its temperature.At what temperature will its root-mean-square speed (thermal speed)be double its value at 20.0°C?
(Multiple Choice)
4.9/5  (40)
(40)
A fixed amount of ideal gas is held in a rigid container that expands negligibly when heated.At 20°C the gas pressure is p.If we add enough heat to increase the temperature from 20°C to 40°C,the pressure will be
(Multiple Choice)
4.9/5  (40)
(40)
The mean free path of an oxygen molecule is 2.0 × 10-5 m,when the gas is at a pressure of 120 Pa and a temperature of 275 K.The molecular mass of oxygen is 32.0 g/mol and the Boltzmann constant is 1.38 × 10-23 J/K,Avogadro's number is 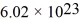 molecules/mole,and the ideal gas constant is
molecules/mole,and the ideal gas constant is 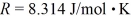 =
= 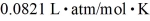 .Assuming that the molecules are moving at their root-mean-square speeds,the average time interval between collisions of an oxygen molecule is closest to
.Assuming that the molecules are moving at their root-mean-square speeds,the average time interval between collisions of an oxygen molecule is closest to
(Multiple Choice)
4.9/5  (49)
(49)
What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948 g/mol and the ideal gas constant is 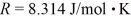 =
= 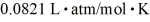 .
.
(Multiple Choice)
4.8/5  (38)
(38)
A sealed 26- 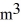 tank is filled with 2000 moles of oxygen gas (O2)at an initial temperature of 270 K.The gas is heated to a final temperature of 460 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is R = 8.314 J/mol • K =
tank is filled with 2000 moles of oxygen gas (O2)at an initial temperature of 270 K.The gas is heated to a final temperature of 460 K.The ATOMIC mass of oxygen is 16.0 g/mol,and the ideal gas constant is R = 8.314 J/mol • K = 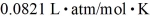 .The final pressure of the gas is closest to
.The final pressure of the gas is closest to
(Multiple Choice)
4.7/5  (38)
(38)
A 25-L container holds ideal hydrogen (H2)gas at a gauge pressure of 0.25 atm and a temperature of 0°C.What mass of hydrogen gas is in this container? The ATOMIC mass of hydrogen is 1.0 g/mol,the ideal gas constant is 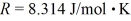 =
= 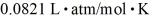 ,and
,and 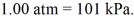
(Multiple Choice)
4.9/5  (36)
(36)
A sample of an ideal gas is slowly compressed to one-half its original volume with no change in temperature.What happens to the average speed of the molecules in the sample?
(Multiple Choice)
4.9/5  (37)
(37)
Dust particles are pulverized rock,which has density 2500 kg/m3.They are approximately spheres 20 μm in diameter.Treating dust as an ideal gas,what is the root-mean-square speed (thermal speed)of a dust particle at 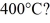 (The Boltzmann constant is
(The Boltzmann constant is 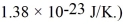
(Multiple Choice)
4.9/5  (35)
(35)
A 0.10- 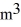 gas tank holds 5.0 moles of nitrogen gas (N2),at a temperature of
gas tank holds 5.0 moles of nitrogen gas (N2),at a temperature of 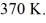 The atomic mass of nitrogen is 14 g/mol,the molecular radius is
The atomic mass of nitrogen is 14 g/mol,the molecular radius is 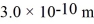 ,and the Boltzmann constant is
,and the Boltzmann constant is 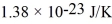 .The root-mean-square speed (thermal speed)of the molecules is closest to
.The root-mean-square speed (thermal speed)of the molecules is closest to
(Multiple Choice)
4.8/5  (39)
(39)
A sealed container holds 0.020 moles of nitrogen (N2)gas at a pressure of 1.5 atmospheres and a temperature of 290 K.The atomic mass of nitrogen is 14 g/mol.The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is 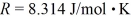 =
= 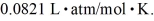 The average translational kinetic energy of a nitrogen molecule is closest to
The average translational kinetic energy of a nitrogen molecule is closest to
(Multiple Choice)
4.9/5  (38)
(38)
The root-mean-square speed (thermal speed)of the molecules of a gas is 200 m/s at a temperature 23.0°C.What is the mass of the individual molecules? The Boltzmann constant is 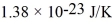 .
.
(Multiple Choice)
4.9/5  (28)
(28)
At 50.0°C,the average translational kinetic energy of a gas molecule is K.If the temperature is now increased to 100.0°C,the average translational kinetic energy of a molecule will be closest to
(Multiple Choice)
4.8/5  (43)
(43)
A cold trap is set up to cause molecules to linger near the suction in a vacuum system.If the cold trap has an effective volume of 0.200 L and is maintained at 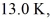 how many molecules are in it at 10.0 Pa of pressure? (Avogadro's number is
how many molecules are in it at 10.0 Pa of pressure? (Avogadro's number is 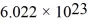 molecules/mol,and the universal gas constant is 8.314 J/mol • K.Assume the behavior of an ideal gas.)
molecules/mol,and the universal gas constant is 8.314 J/mol • K.Assume the behavior of an ideal gas.)
(Multiple Choice)
4.8/5  (27)
(27)
A 5.0-liter gas tank holds 1.7 moles of monatomic helium (He)and 1.10 mole of diatomic oxygen (O2),at a temperature of 260 K.The ATOMIC masses of helium and oxygen are 4.0 g/mol and 16.0 g/mol,respectively.What is the ratio of the root-mean-square (thermal)speed of helium to that of oxygen?
(Multiple Choice)
4.7/5  (38)
(38)
A vertical tube that is closed at the upper end and open at the lower end contains an air pocket.The open end of the tube is under the water of a lake,as shown in the figure.When the lower end of the tube is just under the surface of the lake,where the temperature is 37°C and the pressure is 1.0 × 105 Pa,the air pocket occupies a volume of 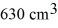 .Suppose now that the lower end of the tube is at a depth of 86 m in the lake,where the temperature is 7.0°C.What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3.
.Suppose now that the lower end of the tube is at a depth of 86 m in the lake,where the temperature is 7.0°C.What is the volume of the air pocket under these conditions? The density of the water in the lake is 1000 kg/m3. 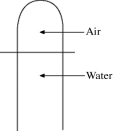
(Short Answer)
4.7/5  (27)
(27)
If we double the root-mean-square speed (thermal speed)of the molecules of a gas,then
(Multiple Choice)
4.9/5  (34)
(34)
What is the mean free path of a gas of randomly-moving hard spheres of radius 2.00 × 10-9 m when the density of spheres is 3.00 × 1019 per cubic meter?
(Multiple Choice)
4.8/5  (39)
(39)
Sometimes an experiment requires a certain pure gas to be used at reduced pressure.One way to achieve this is to purchase a sealed glass container filled with the gas,and to introduce the gas into a vacuum by attaching the glass container to the vacuum chamber and breaking the tip of the glass container using a metallic bean and a magnet.If the volume of the glass container is 1.0 L and it is at a pressure of 1.0 × 105 Pa and if the vacuum chamber has a volume of 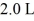 ,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
,what will the pressure be after the gas,which is to be assumed to be an ideal gas,is released into the vacuum chamber and the temperature has returned to its original value? (Note that the glass container remains part of the system.)
(Multiple Choice)
4.9/5  (43)
(43)
What is the average kinetic energy of an ideal gas molecule at 569°C? (The Boltzmann constant is 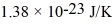 .)
.)
(Multiple Choice)
4.7/5  (39)
(39)
Showing 21 - 40 of 58
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)